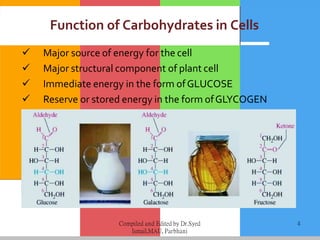
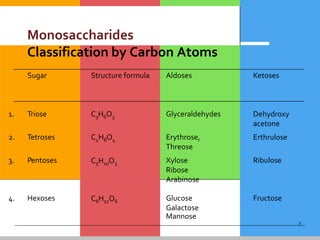
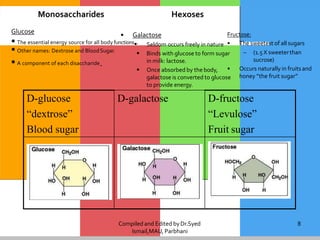
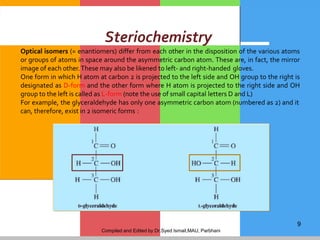
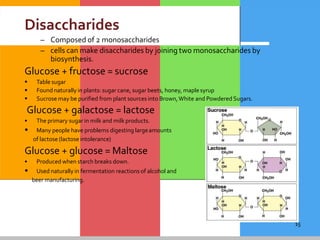
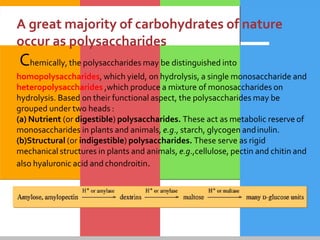
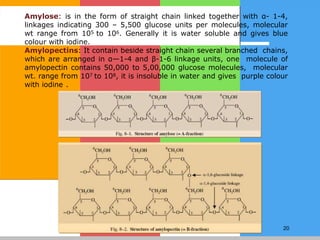
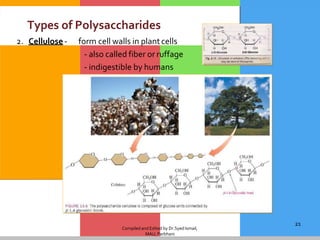
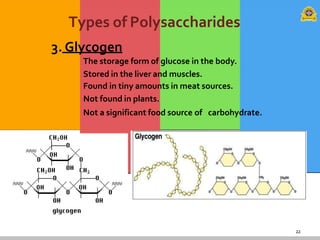
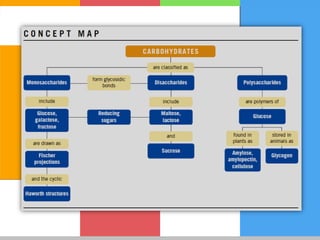
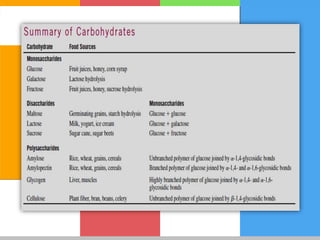
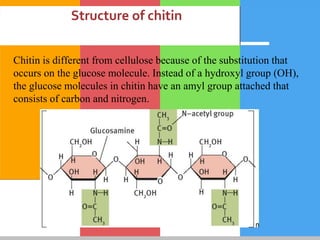
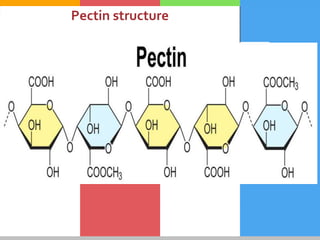
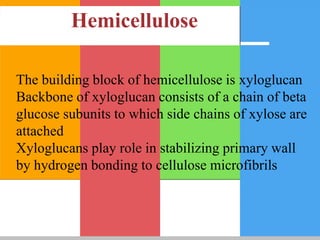
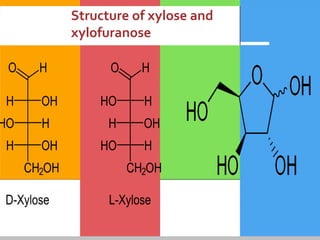
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen that serve as a major energy source. They include monosaccharides (simple sugars), oligosaccharides (short-chain sugars), and polysaccharides (long-chain sugars). Monosaccharides like glucose are the basic units that link together to form more complex carbohydrates. Polysaccharides provide structure and storage functions, with cellulose giving structure to plants and glycogen storing glucose in animal tissues. Carbohydrates serve vital energy, structural, and storage roles across living organisms.