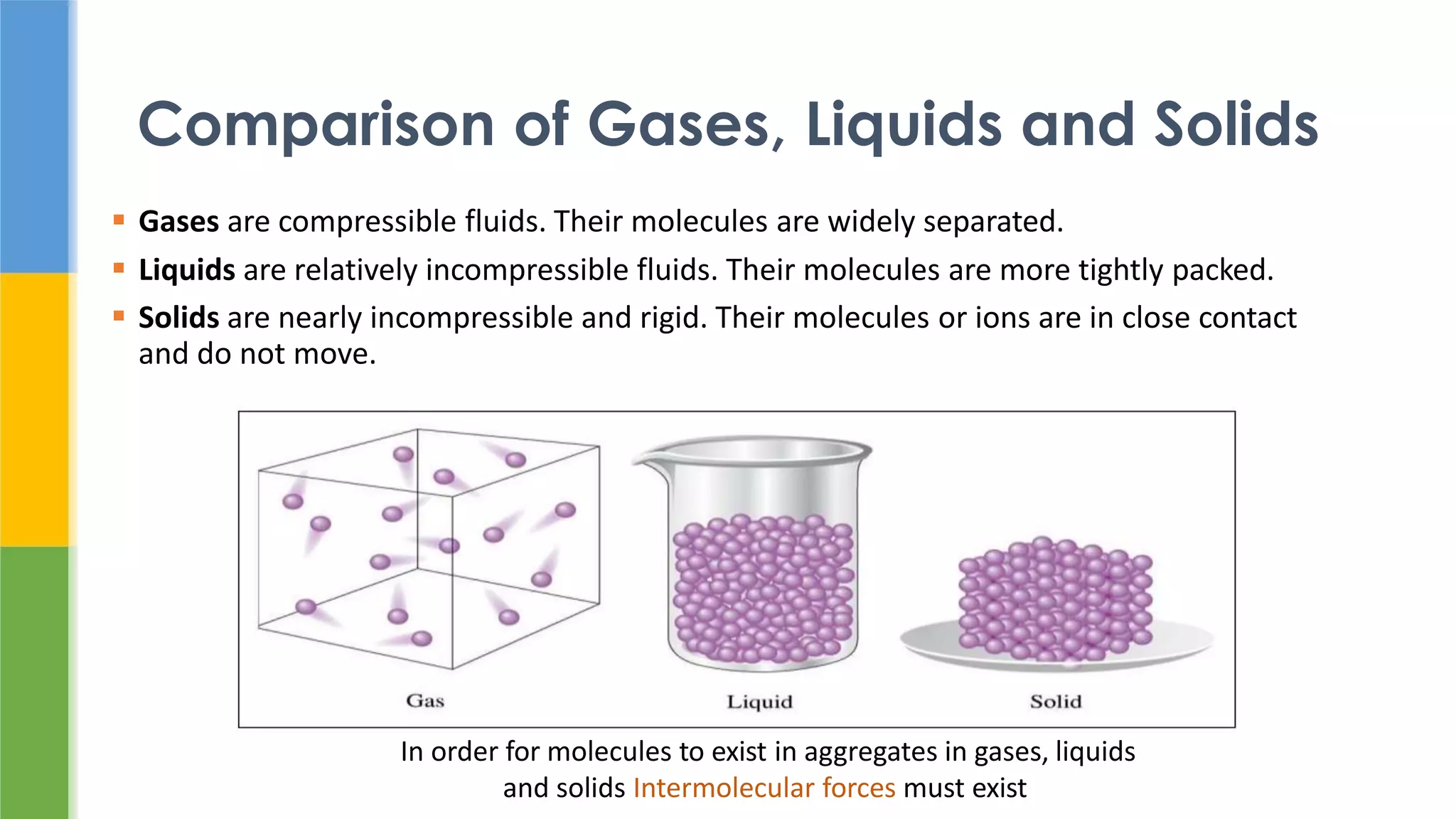
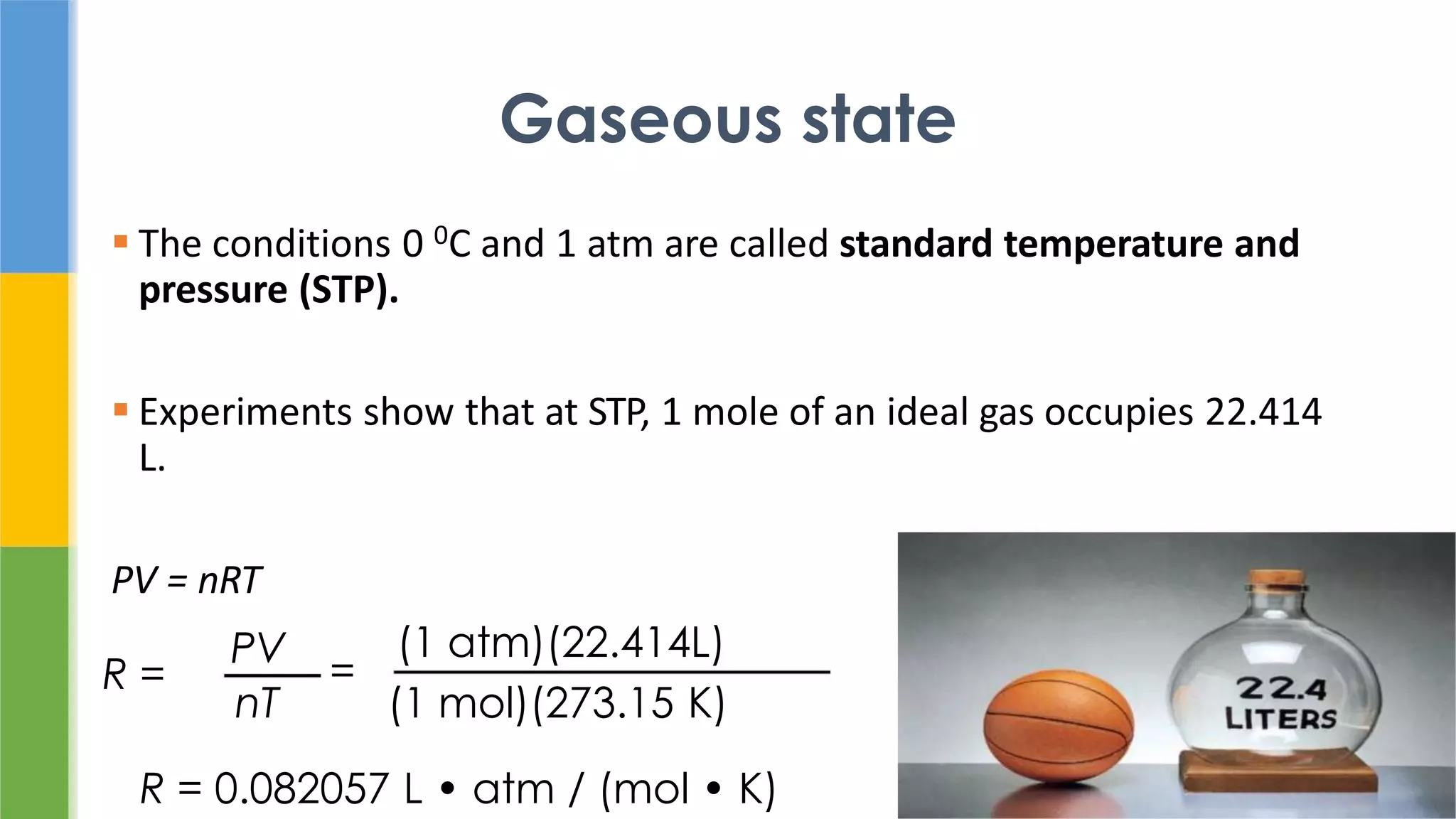
The document discusses states of matter and pharmaceutical materials. It begins by comparing gases, liquids, and solids, noting that solids have molecules in close contact that do not move. It then discusses intermolecular forces, ideal gas laws, liquefaction of gases, and the solid state including crystals, unit cells, polymorphism, and amorphous solids. It notes that polymorphism can impact properties like solubility, melting point, and bioavailability which are important for pharmaceutical processes and drug performance.
















































































![Study Questions
Define the following terms:
[solid, liquid, gas, pure substance, compound, mixture, element, heterogeneous mixture, homogeneous mixture,
extensive properties, intensive properties, chemical properties, physical properties, density, color, texture, conductivity,
malleability, ductility, boiling point, melting point, flammability, corrosiveness, volatility, pounding, tearing, cutting,
dissolving, evaporating, fermenting, decomposing, Exothermic, endothermic, mass, density, gravity, adhesive force,
cohesive force, etc]
Respond to the following questions:
➢What is a mass, inertia, and how do these two variables affect the movement of material substance
➢What is gravity and how does it affect the movement of material substance
➢Give a descriptive account of the phases of matter with logical relevance to state of medicines as they are taken
for their respective therapeutical values
➢What is viscosity and its relation with fluids
➢What is surface tension and association with activities a substance material with surface area
➢Describe some key phase changes of materials substance when exposed to some environmental conditions of
change
➢How is a chemical change different from a physical change
Group work discussional questions:
➢ Give a detailed description Binding Forces Between Molecules
➢ Explain what is meant by Repulsive and Attractive Forces
➢ Explain with examples The Gaseous State of pharmaceutical material
➢ Explain with examples The solid State of pharmaceutical material
➢ Explain with examples The Crystalline State of pharmaceutical material
➢ Explain with examples The liquid State of pharmaceutical material
➢ Explain with examples The semi-solid State of pharmaceutical material
➢ Explain with examples The semi-solid State of pharmaceutical material
➢ Explain with examples The semi-liquid State of pharmaceutical materia](https://image.slidesharecdn.com/1-2-physicalstateofmatter-221124230616-d3fe2aec/75/1-2-PhysicalState-of-Matter-pdf-83-2048.jpg)