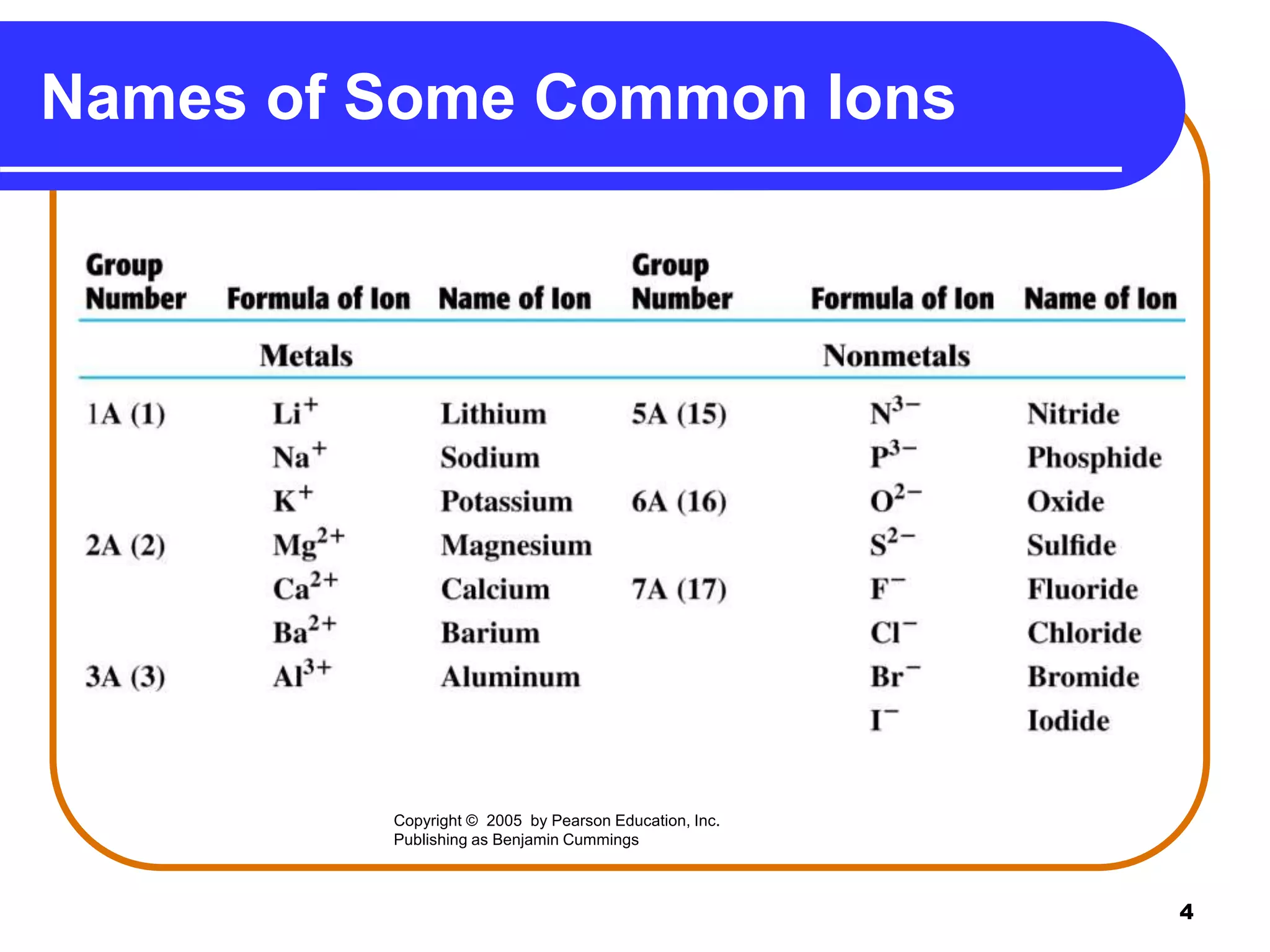
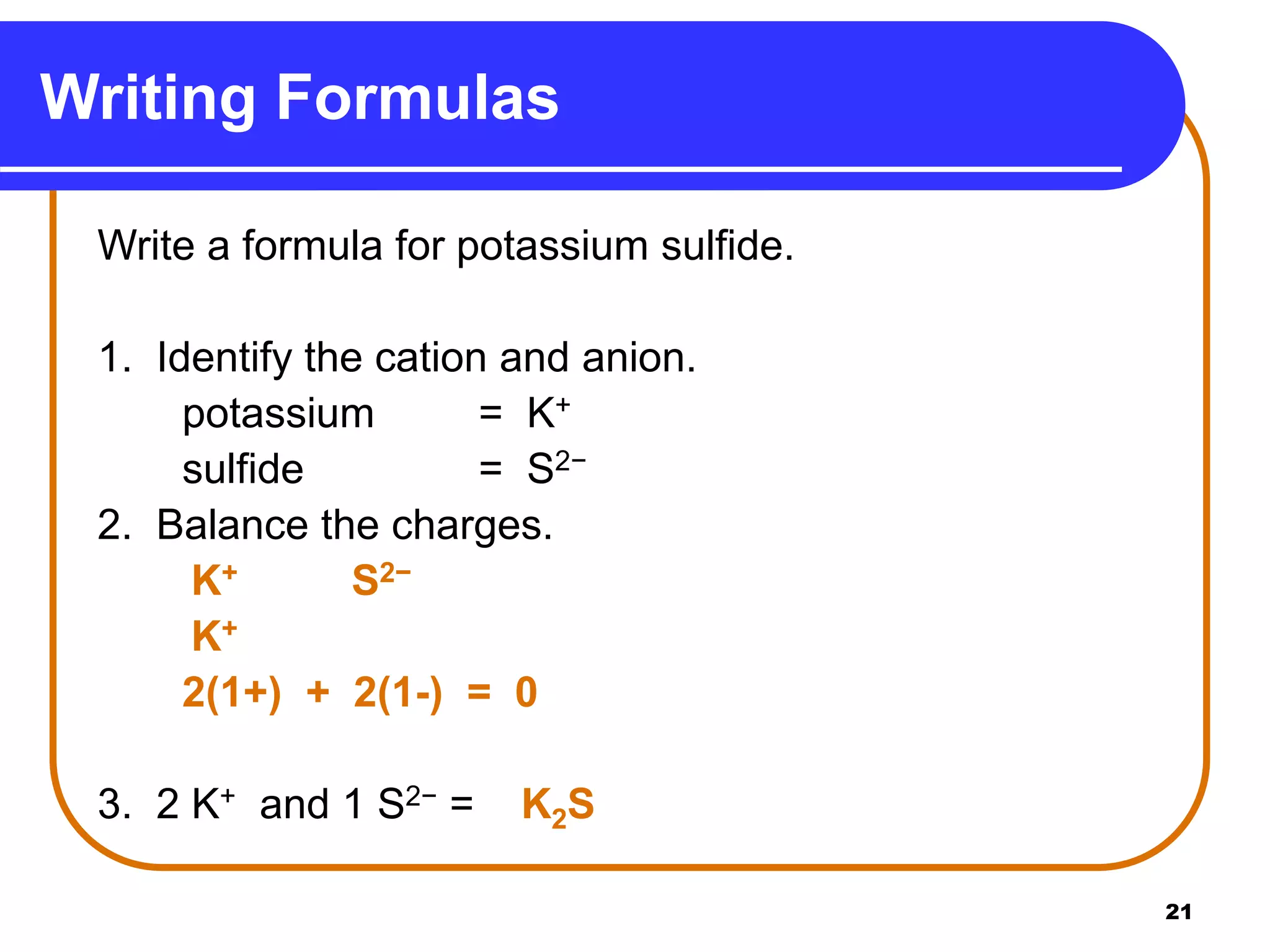
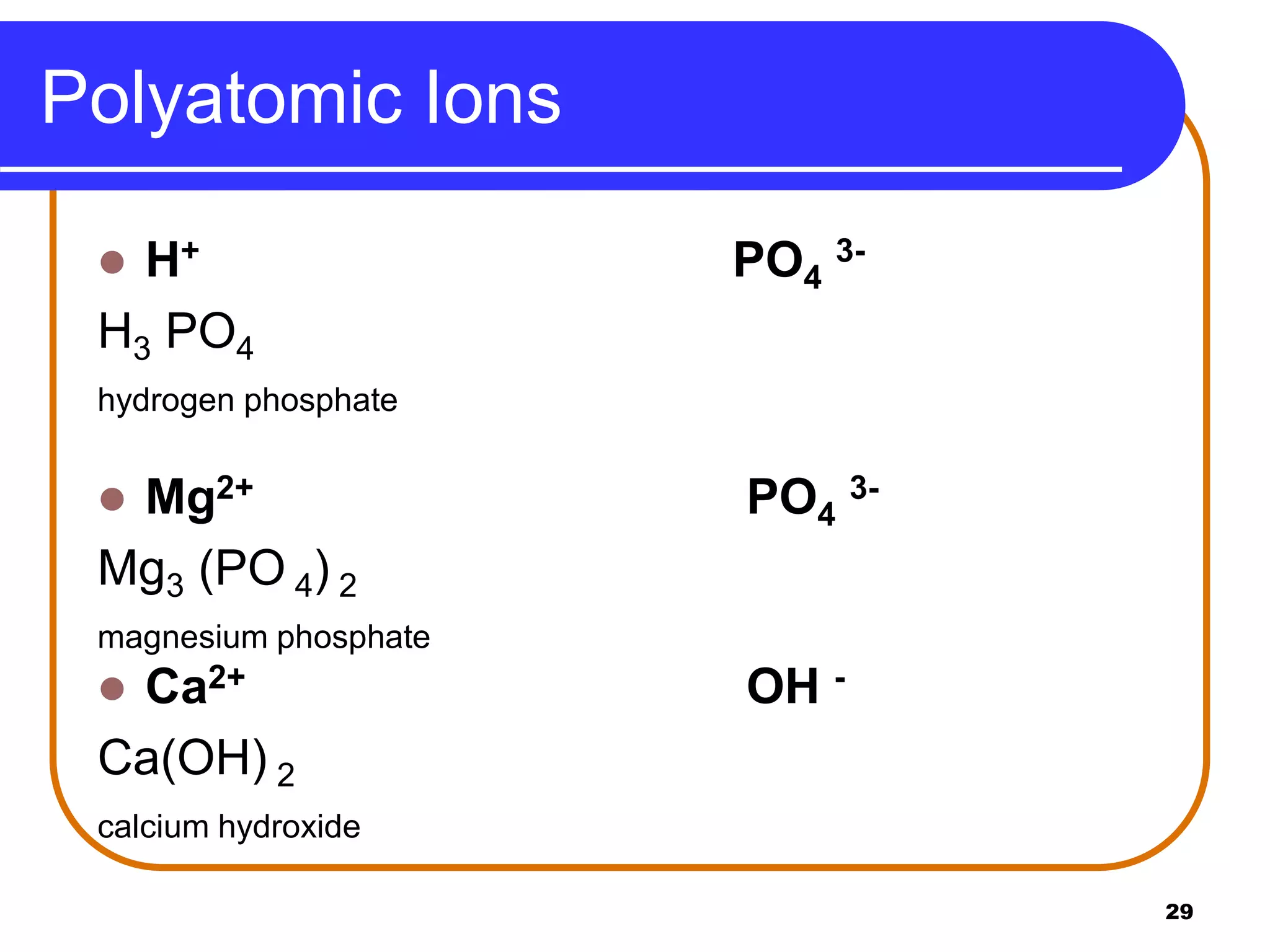
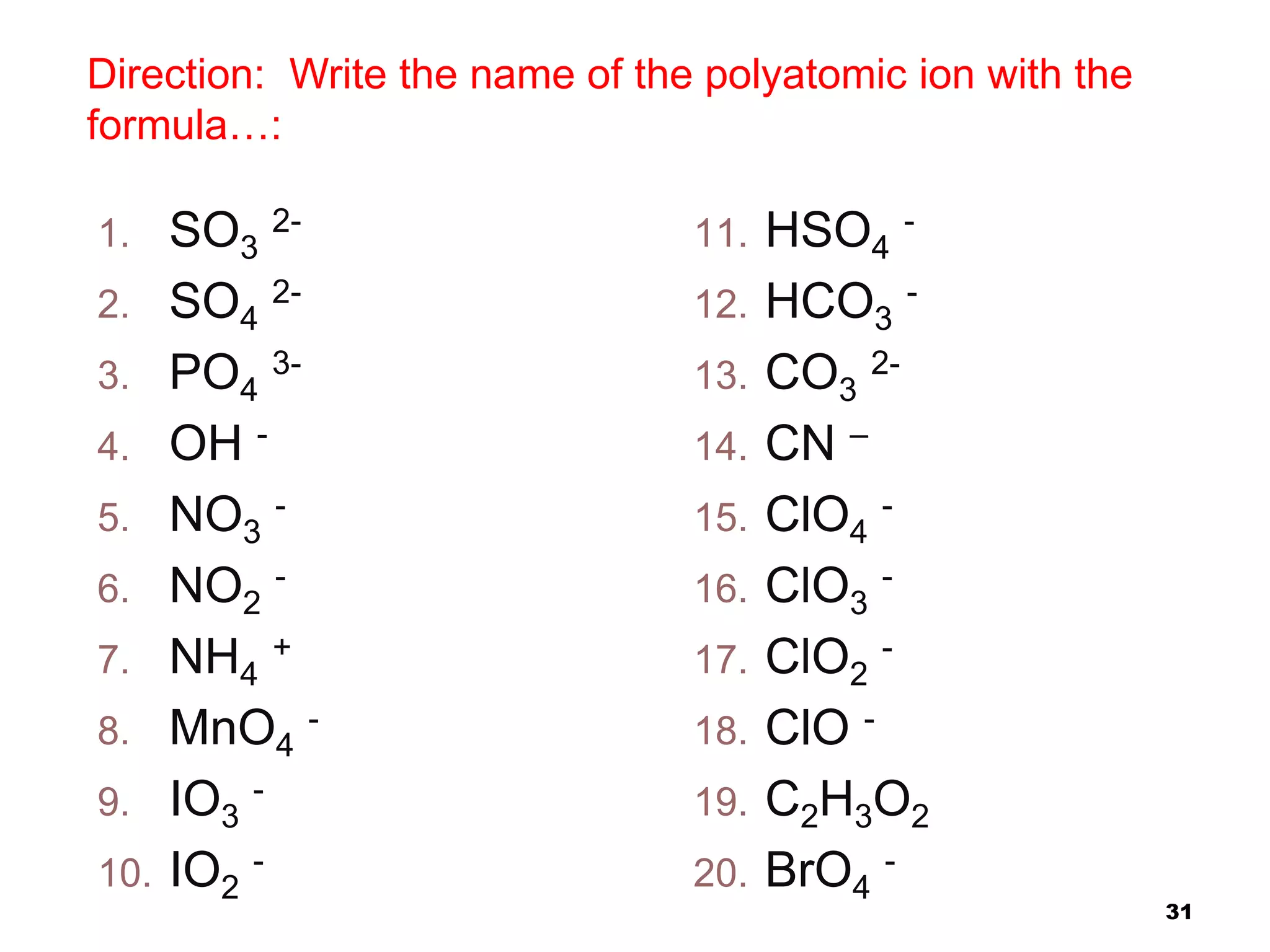
This document provides information on naming ionic and molecular compounds. It discusses how to name ionic compounds containing two elements by identifying the cation and anion. It also discusses how to name compounds containing transition metals with variable charge by including the ionic charge in Roman numerals. The document shows examples of writing formulas from compound names and vice versa. It introduces molecular compound naming using prefixes to indicate the number of atoms of each element. Finally, it discusses naming polyatomic ions based on their chemical formulas.