Embed presentation
Download to read offline

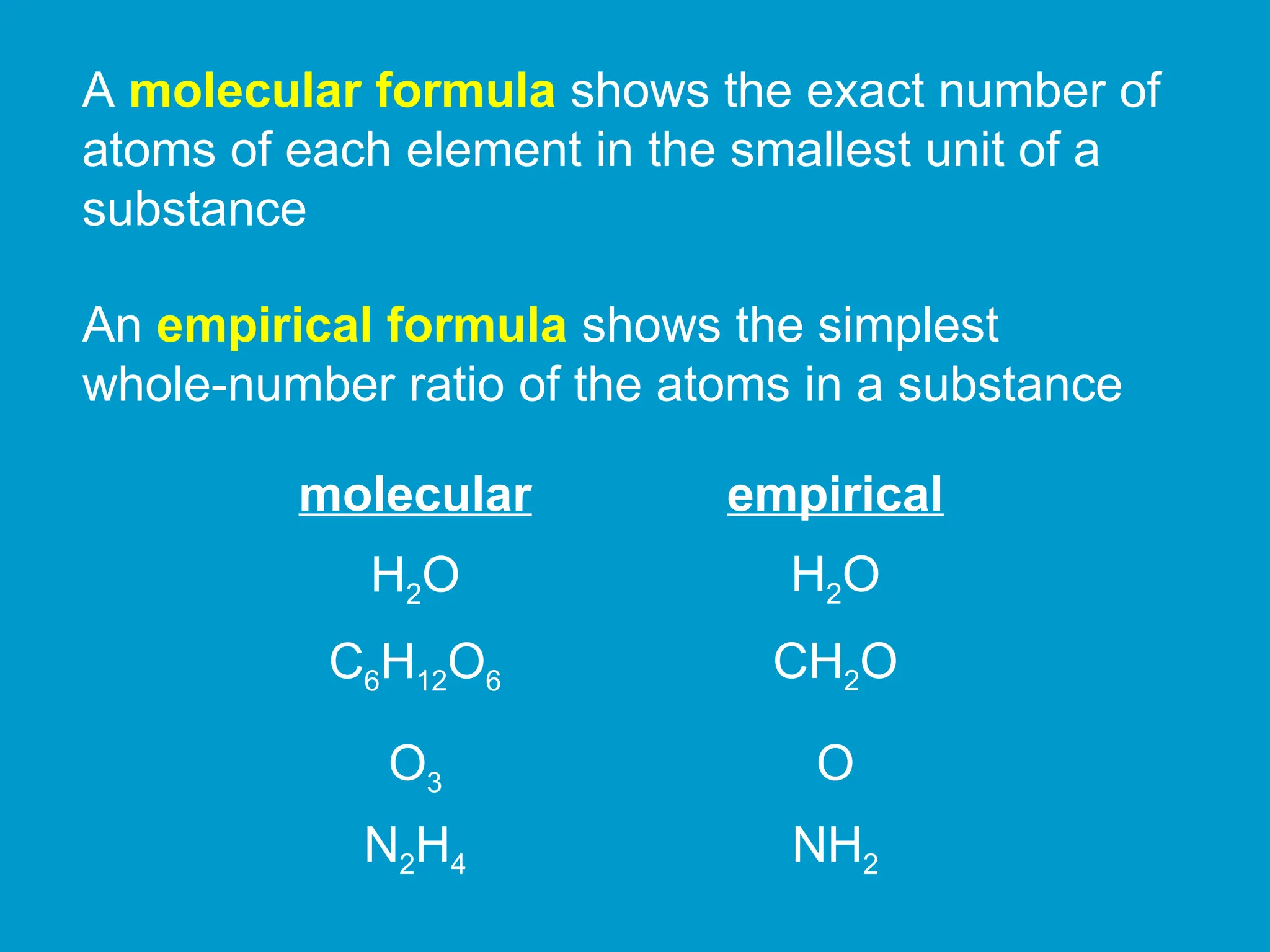
The document outlines guidelines for writing chemical formulas for binary compounds, indicating that the element farther left in the periodic table is listed first, with specific rules for the placement of hydrogen. It distinguishes between molecular formulas, which display the exact number of atoms, and empirical formulas, which show the simplest whole-number ratio of the atoms. Examples include compounds like KCl and H2O to illustrate these rules.

