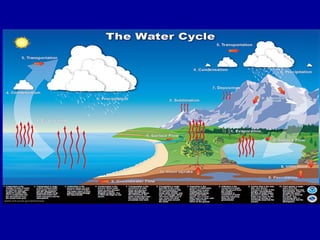
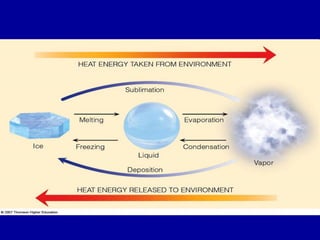
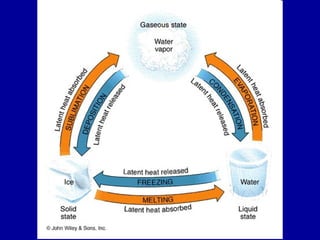
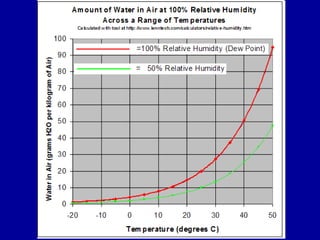
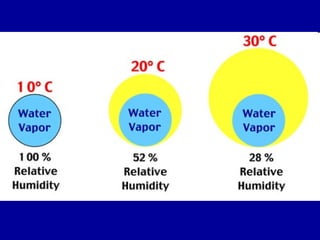
This document discusses various aspects of water in the atmosphere and how it relates to meteorology. It covers the water cycle and how water changes state between solid, liquid, and gas through processes like melting, evaporation, condensation, and sublimation. It also defines key humidity terms like relative humidity, saturation, and dew point. Relative humidity depends on both the air temperature and its water vapor content compared to the maximum it can hold at a given temperature.