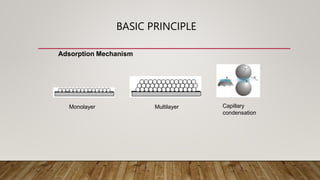
The document discusses Brunauer–Emmett–Teller (BET) surface area analysis, which uses physical adsorption of gas molecules on a solid surface to determine the surface area and pore size distribution of a material. It describes the BET theory of multilayer adsorption, which extended the Langmuir model. The instrumentation involves a removable sample cell, dosing manifold, pressure transducers, and vacuum system. Sample preparation requires cleaning and degassing the sample. Results obtained from the instrument include isotherms and the BET linear equation used to calculate surface area.