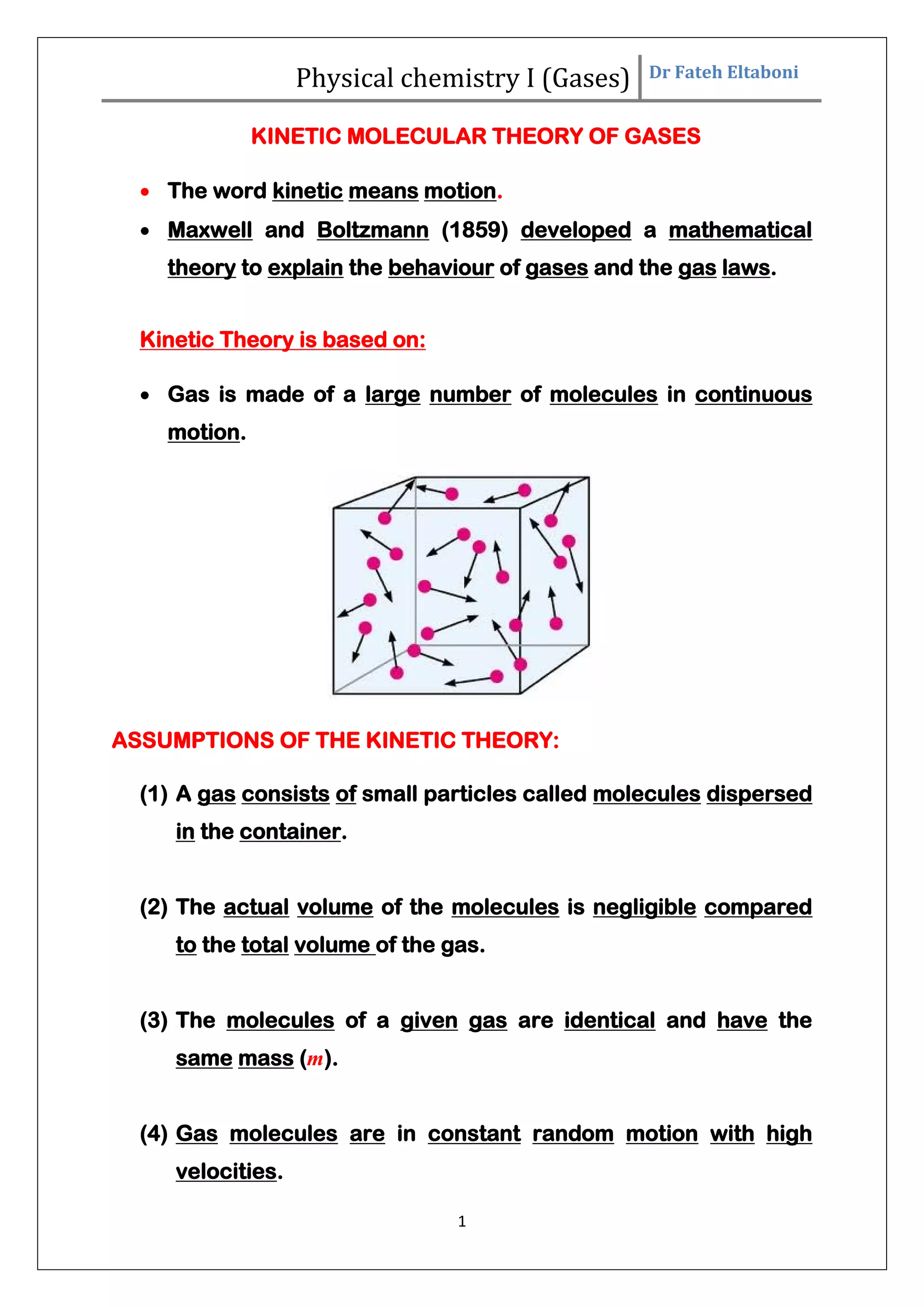
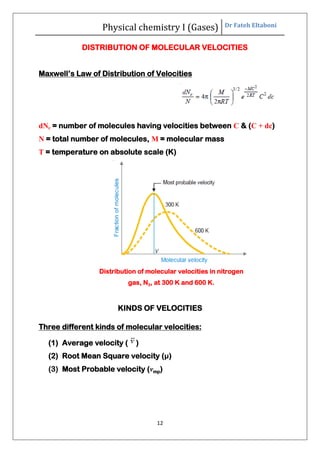
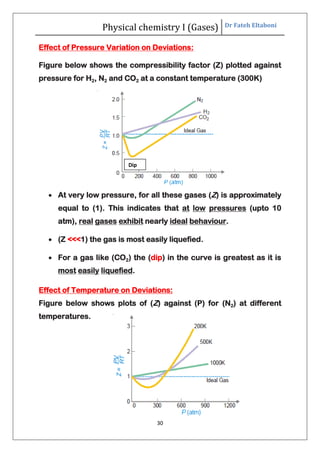
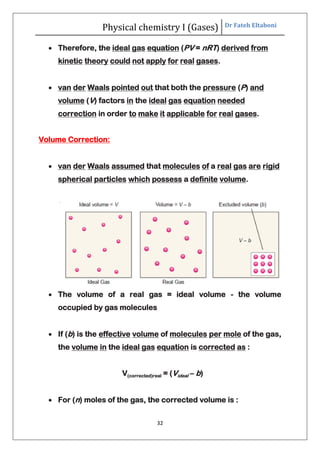
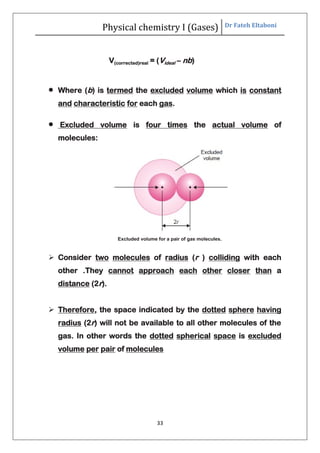
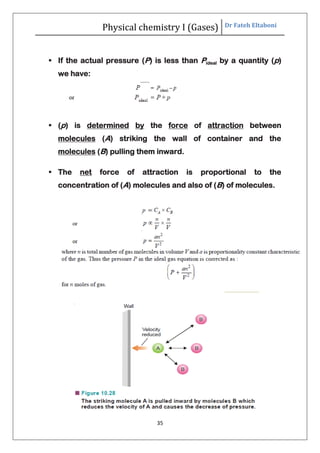
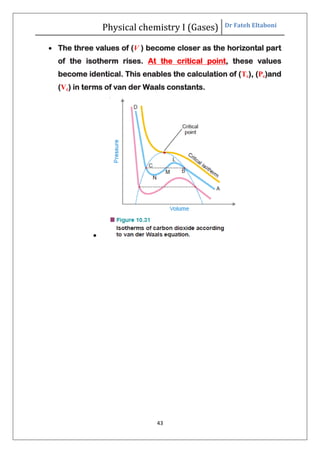
The document summarizes the kinetic molecular theory of gases. It describes the assumptions of the theory, including that gases are made of molecules in constant random motion. It defines key terms like average velocity, root mean square velocity, and most probable velocity. The derivation of the kinetic gas equation from molecular collisions is shown. The gas laws of Boyle, Charles and Avogadro are deduced from the kinetic gas equation. Deviations from ideal gas behavior at high pressures or low temperatures are also summarized.