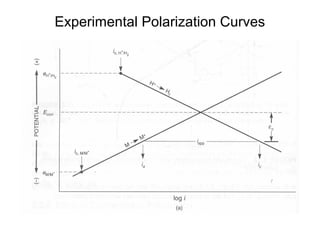
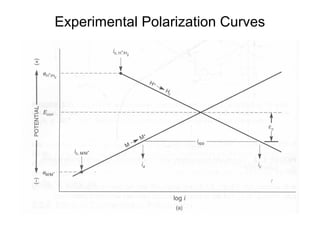
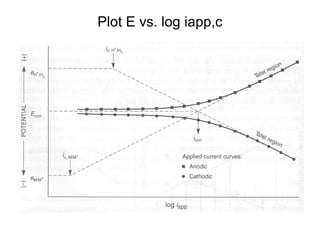
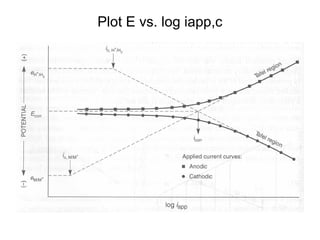
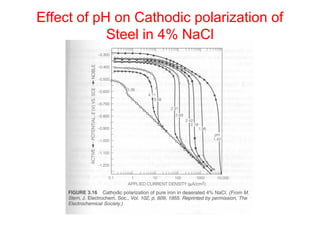
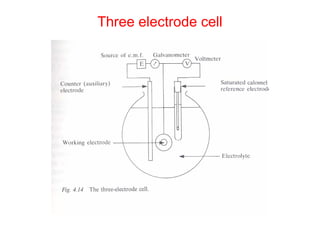
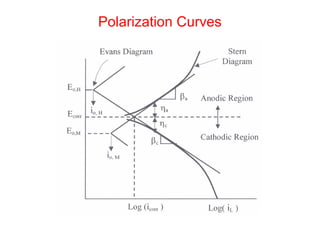
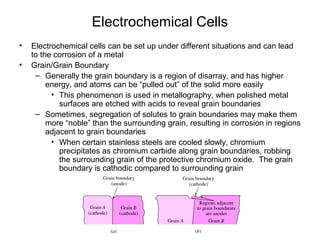
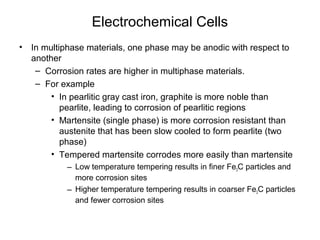
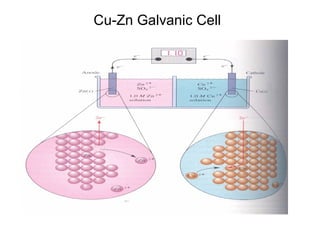
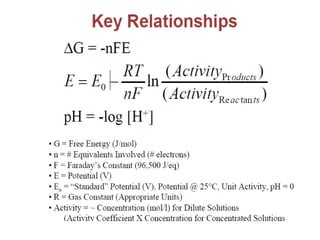
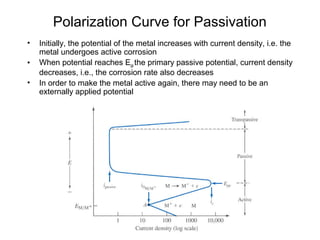
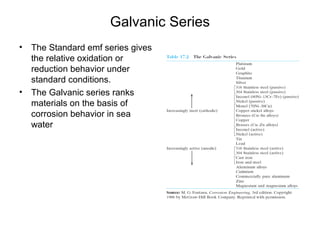
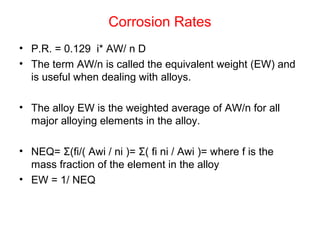
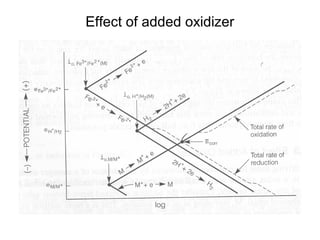
This document summarizes lecture material on corrosion kinetics. It discusses various types of electrochemical cells that can lead to corrosion, including grain boundaries and multiphase materials. It also covers polarization, passivation, galvanic series, corrosion rates, concentration polarization, and experimental polarization curves. Key points include how concentration gradients can limit corrosion current and affect polarization, and how polarization curves are used to determine corrosion kinetics parameters.


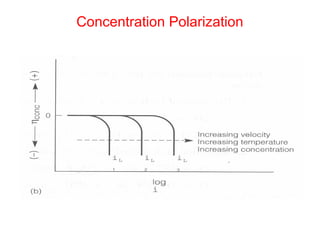
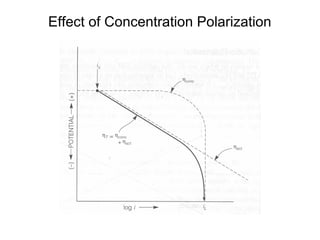
![Concentration Polarization
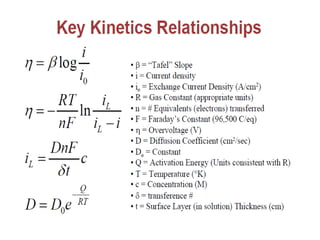
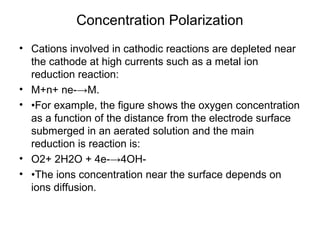
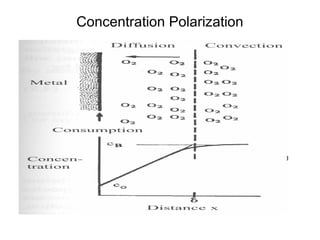
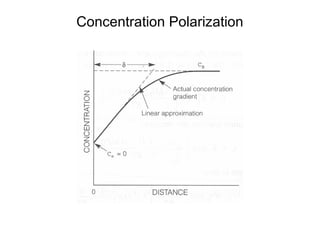
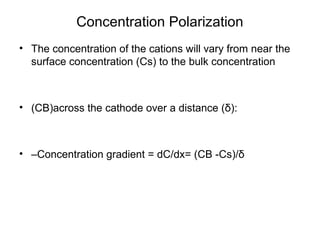
• M+n+ ne-→M
• •Before current flow:
• –ei=eo+ 2.3RT/nF log ([CB]/1)
• •After current flow:
• –ef=eo+ 2.3RT/nF log ([Cs]/1)
• •Concentration cathodic overpotential = ηc,conc
• •= ef-ei= 2.3RT/nF*log (Cs/CB)
• •Cs= CB-icδ/(nFDions)
• •CB= iLδ/(nFDions)
• •ηc,conc= 2.3RT/nF Log {1-(ic/il) }
• –If ic<<il, then ic/il→0 and ηc,conc= 2.3RT/nF log (1) = 0
• –If ic≈il, then ic/il→1 and ηc,conc= 2.3RT/nF log (0) = -∞](https://image.slidesharecdn.com/lecture3corrosionkinetics-160324132559/85/Lecture-3-corrosion-kinetics-15-320.jpg)
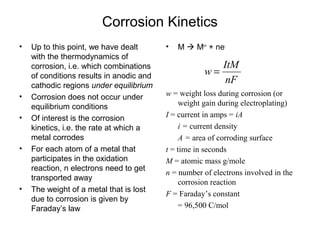
![Concentration Polarization
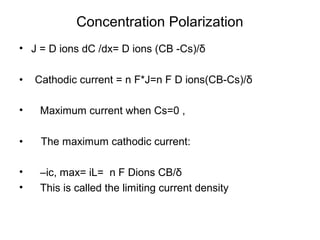
• Q: Estimate iL at 298 K for copper
reduction reaction with bulk concentration
of [Cu++] = 0.1 M in unstirred solution
where δ=0.3 mm. DCu++= 7x10-10m2/s
• •iL= nFDionsCB/δ= 2*96500* 7x10-
100.1/0.0003 = 4.58 mA/cm2](https://image.slidesharecdn.com/lecture3corrosionkinetics-160324132559/85/Lecture-3-corrosion-kinetics-20-320.jpg)



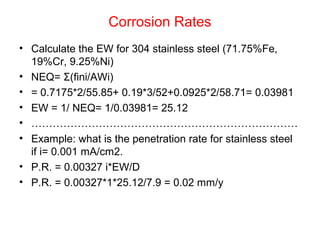
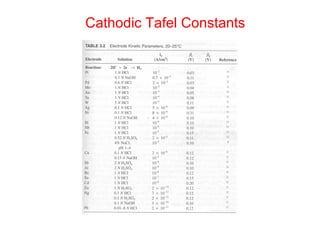
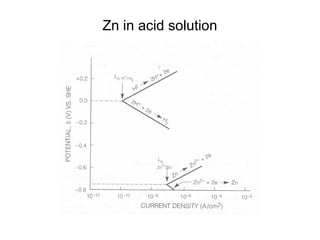
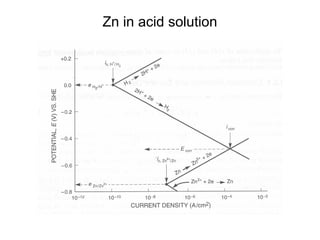
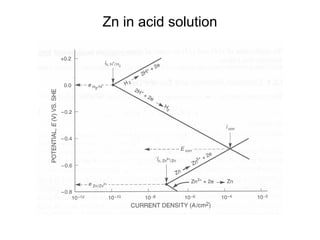
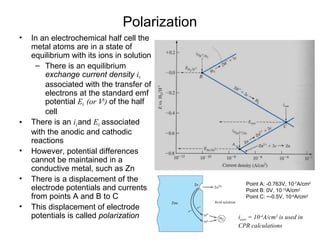
![Example:
• Q: Estimate the corrosion rate, penetration rate, and
Ecorrfor a Zn tank containing oxygen free solution
(pH=0). Assume both anodic and cathodic reactions are
under activation control. [Zn++] = 1 M at 298 K.
• •io, Zn= 1x10-7A/cm2, βa= 0.09 V/decade
• •io, H= 1x10-10A/cm2 , βc= -0.08 V/decade](https://image.slidesharecdn.com/lecture3corrosionkinetics-160324132559/85/Lecture-3-corrosion-kinetics-37-320.jpg)


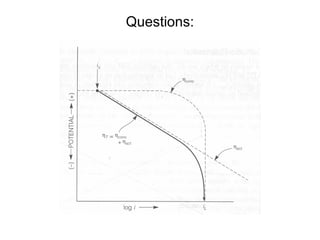
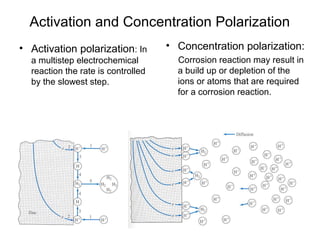
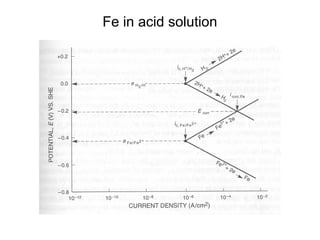
![Questions:
• Estimate the corrosion rate, penetration rate, and
Ecorrfor steel tank containing aerated acid (pH=2).
Assume both anodic and cathodic reactions are under
activation control. [Fe++] = 0.1 M at 298 K.
• •io, Fe= 1x10-6A/cm2, βa= 0.1 V/decade
• •io, H= 1x10-5A/cm2 , βc= -0.1 V/decade](https://image.slidesharecdn.com/lecture3corrosionkinetics-160324132559/85/Lecture-3-corrosion-kinetics-41-320.jpg)