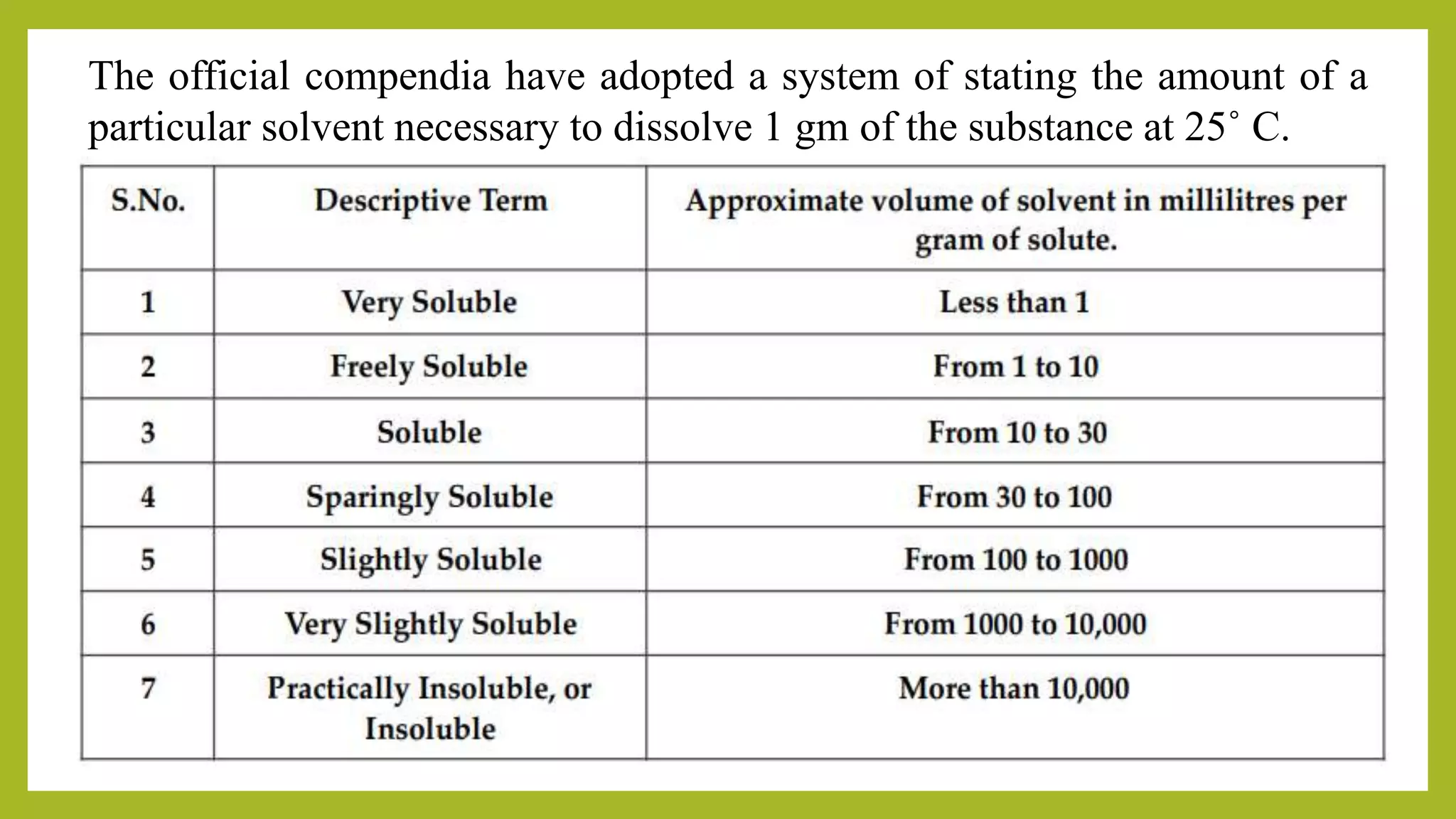
Kota College of Pharmacy discusses various methods of expressing the concentration of solutions. Concentration can be expressed in terms of percent by weight/weight (%w/w), percent by weight/volume (%w/v), percent by volume/volume (%v/v), molarity (M), molality (m), normality (N), and formality (F). A saturated solution is one that has dissolved all the solute it is capable of holding at a given temperature, which is typically assumed to be 25°C unless otherwise specified. Common examples of concentration expressions and saturated solutions are provided.