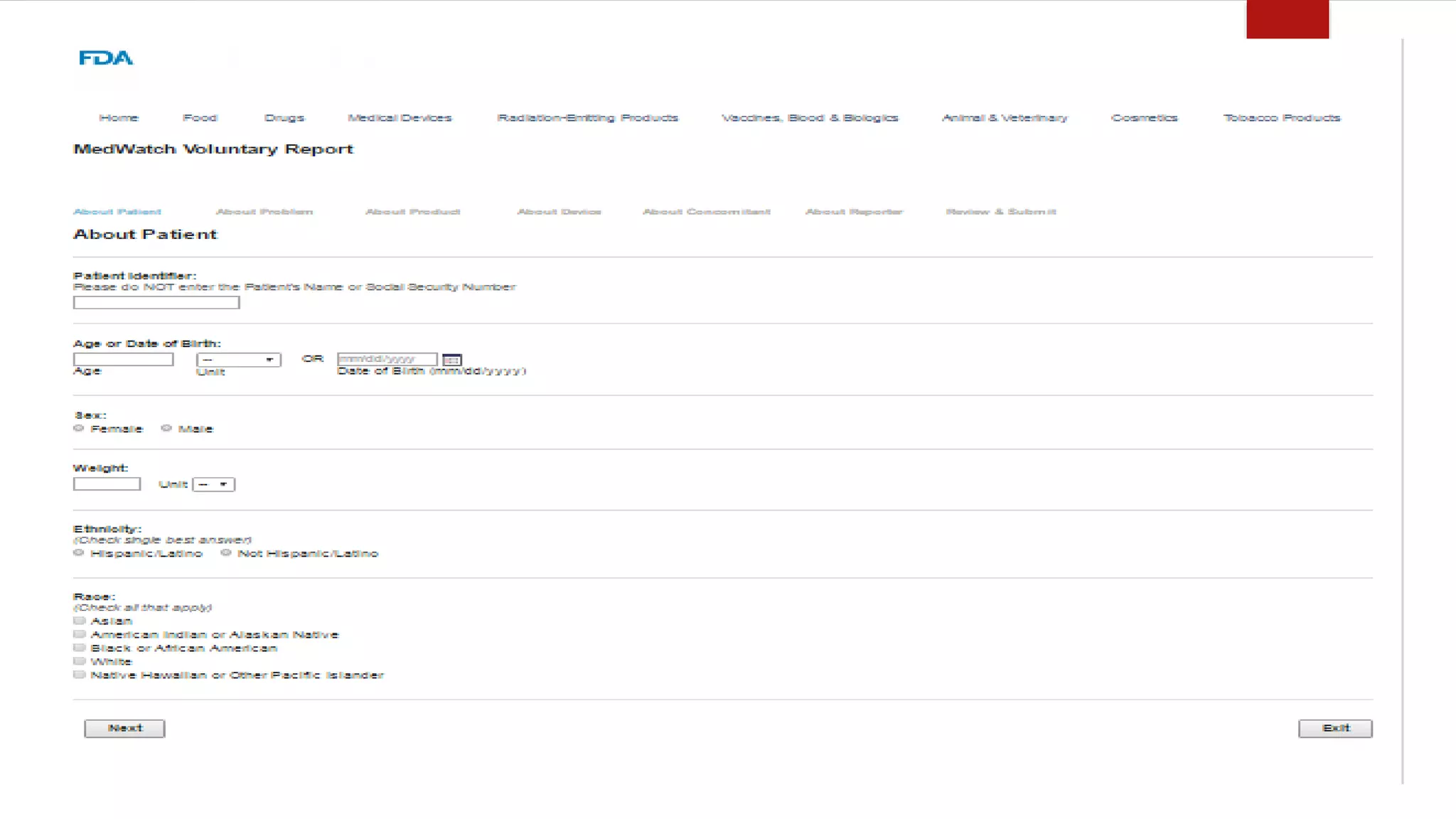
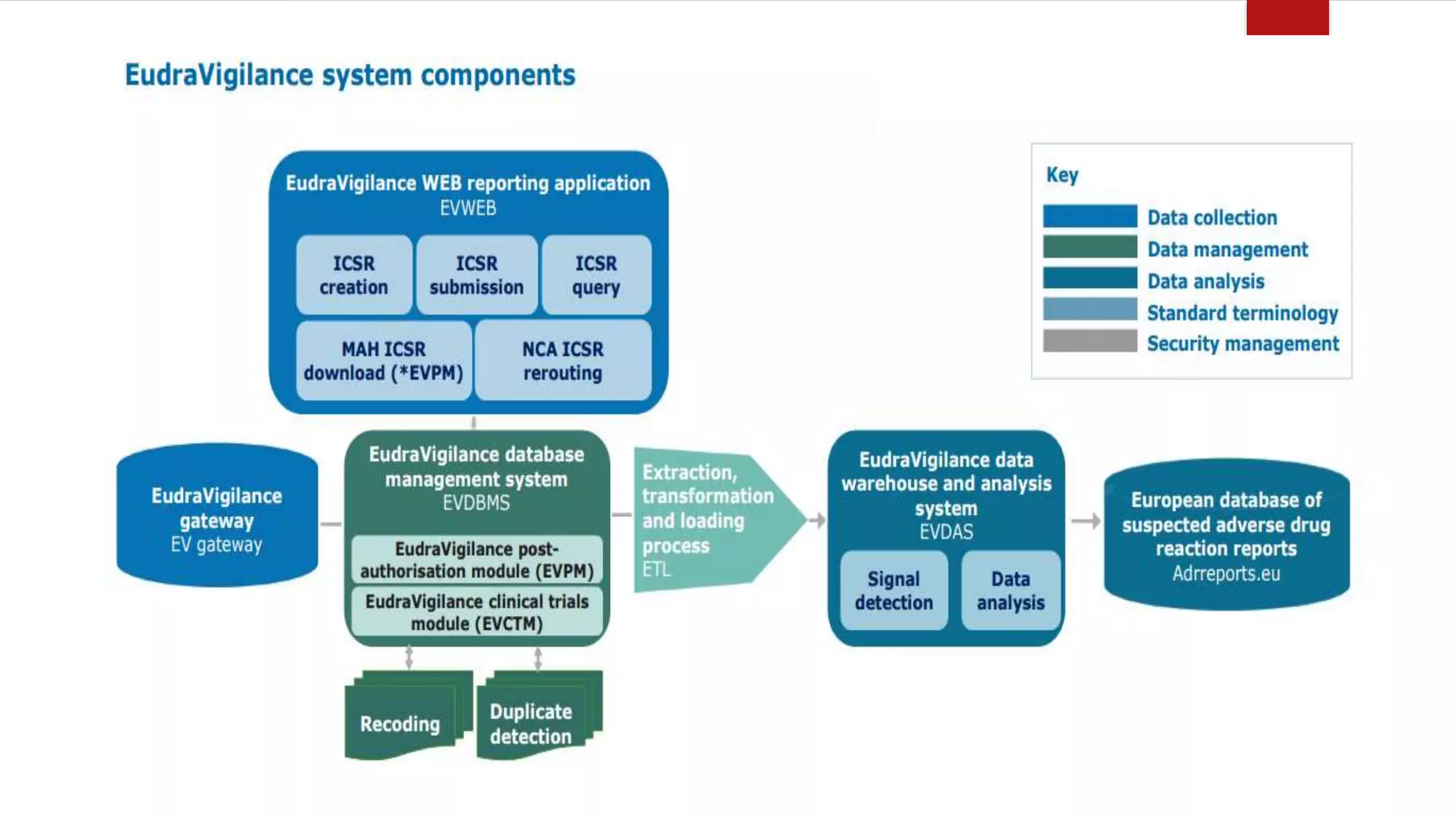
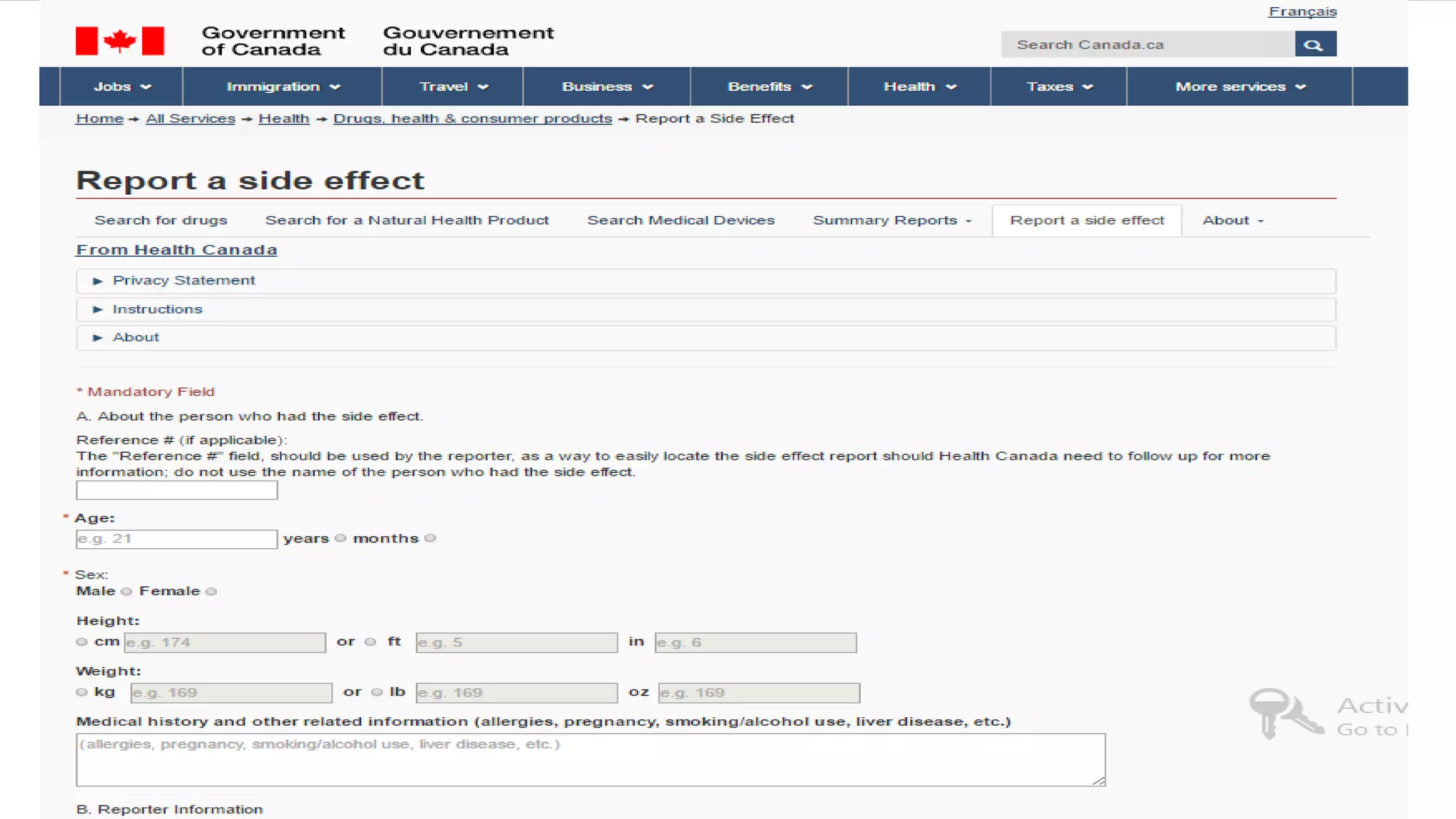
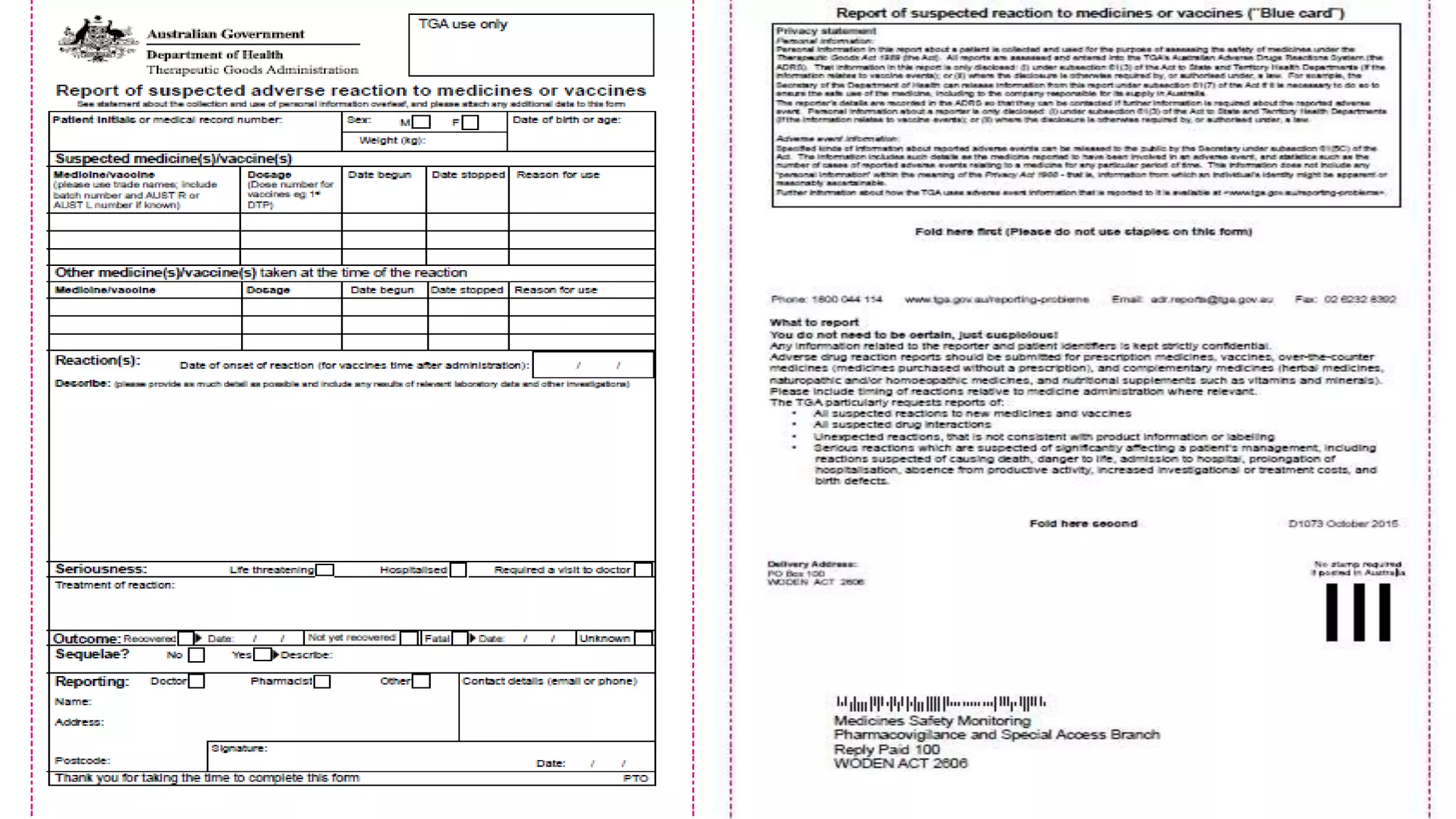
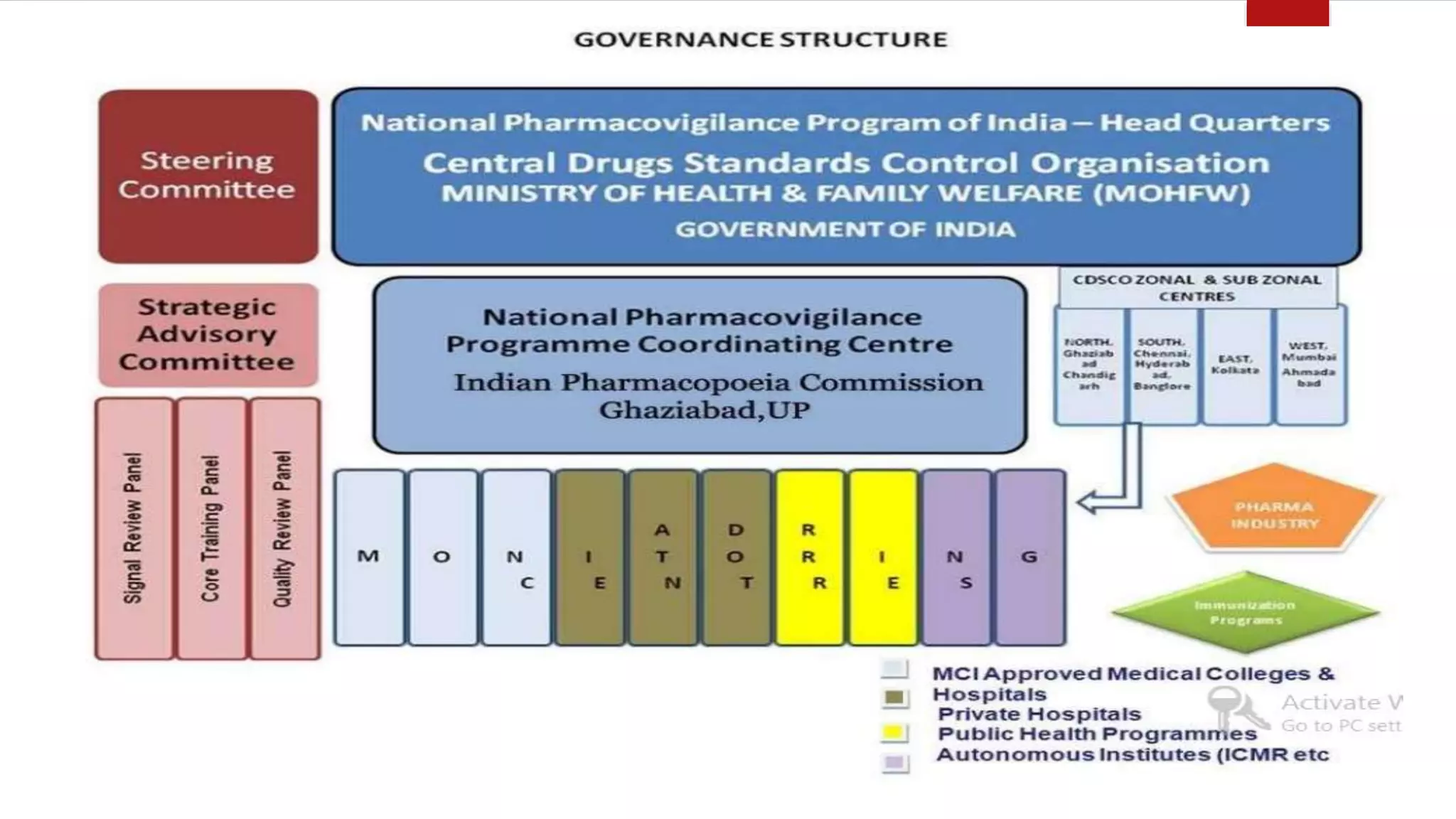
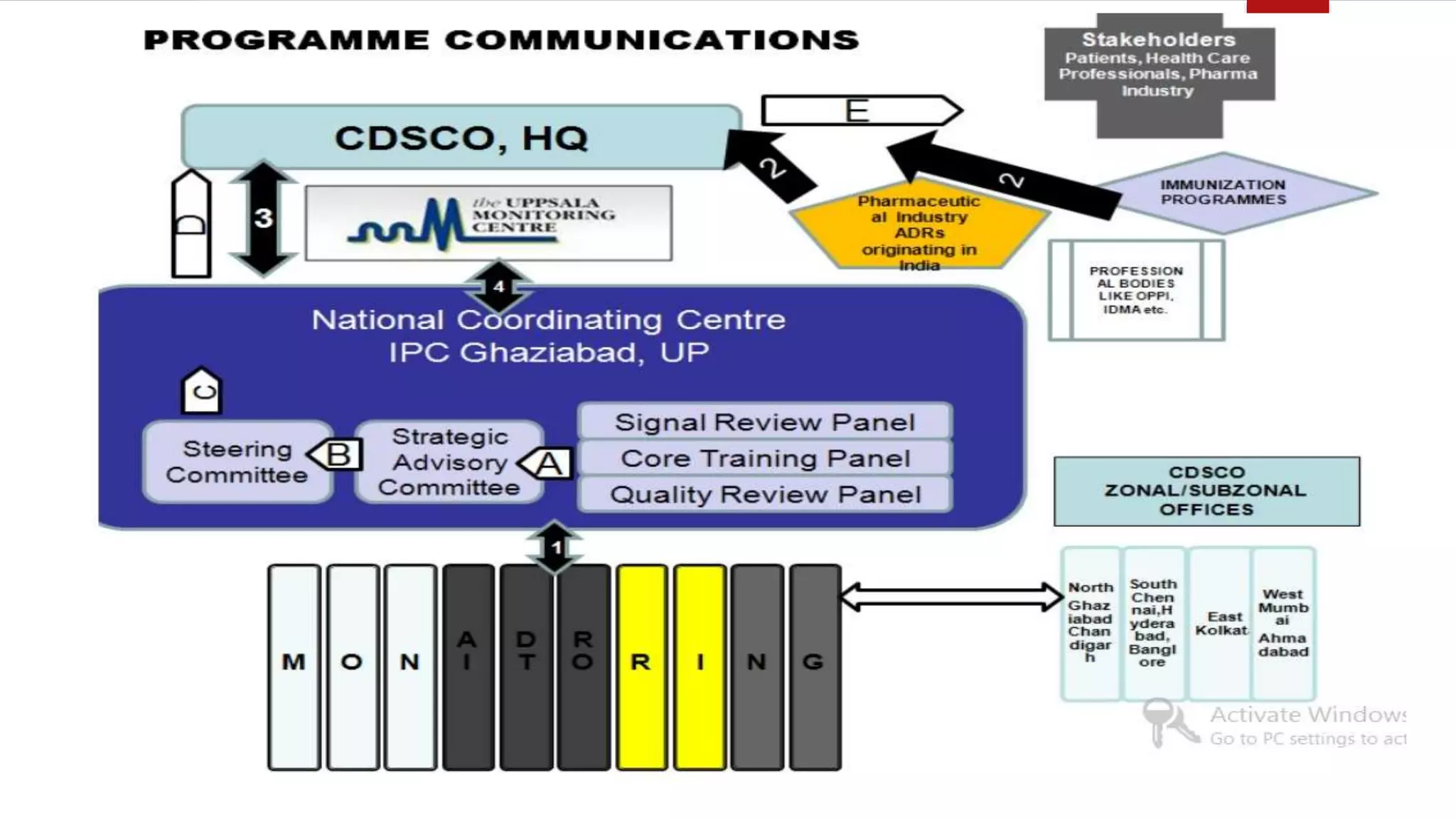
The document discusses various pharmacovigilance systems and methodologies used around the world to monitor the safety of medicines. It describes the WHO global database VigiBase which contains reports of adverse drug reactions. It also outlines other pharmacovigilance systems like EudraVigilance in Europe, FAERS in the US, Canada Vigilance in Canada, and the Yellow Card Scheme in the UK. The document then discusses data mining and analysis techniques used for pharmacovigilance, including those for pre-marketing surveillance using computational approaches as well as those for post-marketing surveillance using spontaneous reporting systems, electronic health records, and non-traditional data sources.