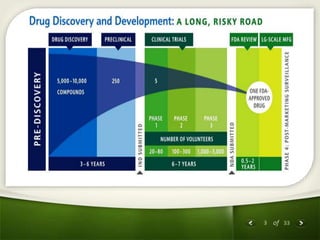
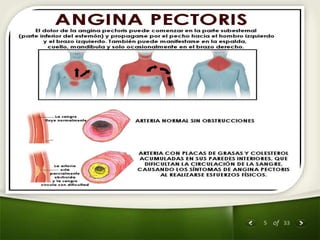
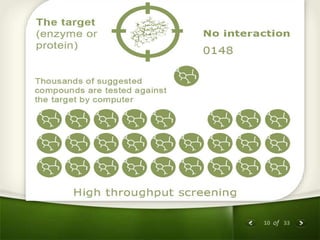
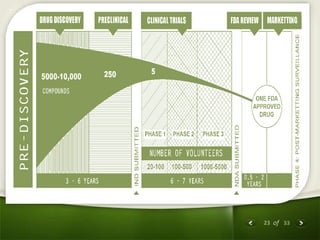
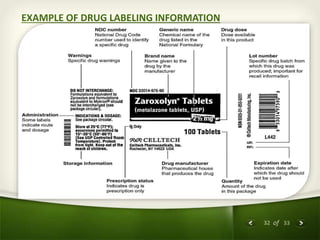
The document summarizes the process of drug discovery and development. It involves several long steps: understanding the disease, finding a biological target, discovering a lead compound through screening or nature, conducting preclinical testing on animals, and then clinical trials in three phases with humans to test safety and efficacy before the FDA decides whether to approve the drug. The entire process from discovery to approval takes an average of 10-15 years and costs $1-2 billion. Drugs also have different categories depending on how they are regulated and prescribed.