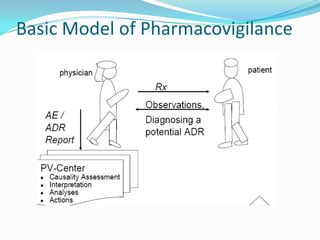
The document discusses various methods used in pharmacovigilance including spontaneous reporting systems, case series, stimulated reporting, active surveillance methods like sentinel sites and drug event monitoring, use of registries, observational studies like cross-sectional, case-control and cohort studies, targeted clinical investigations and descriptive studies. It also outlines the key aims and shared responsibilities of pharmacovigilance among drug companies, regulatory authorities, doctors, pharmacists and nurses.