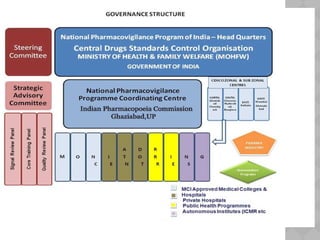
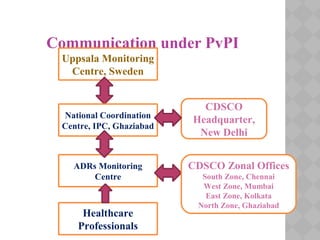
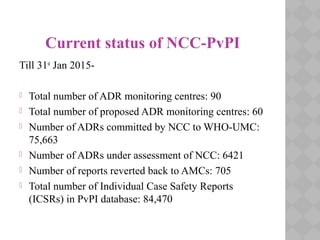
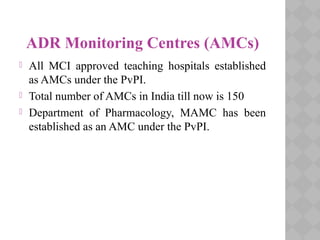
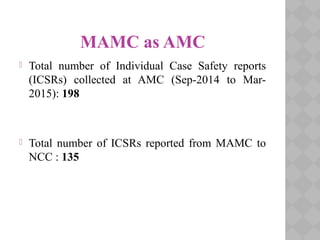
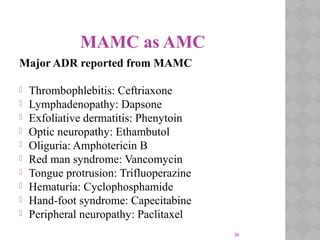
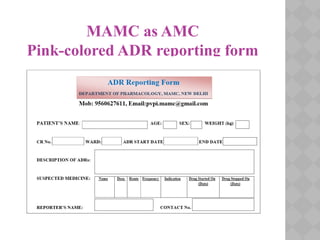
Pharmacovigilance Programme of India (PvPI) was initiated in July 2010 to monitor adverse drug reactions in India. It is coordinated by the Central Drugs Standard Control Organization and has a National Coordination Centre located at the Indian Pharmacopoeia Commission. The objectives of PvPI are to create a nationwide patient safety reporting system, analyze benefit-risk ratios of medicines, and support regulatory decision making. MAMC has established an ADR Monitoring Centre as part of PvPI, which has reported over 135 cases and monitors reactions to drugs like ceftriaxone, dapsone, and phenytoin.