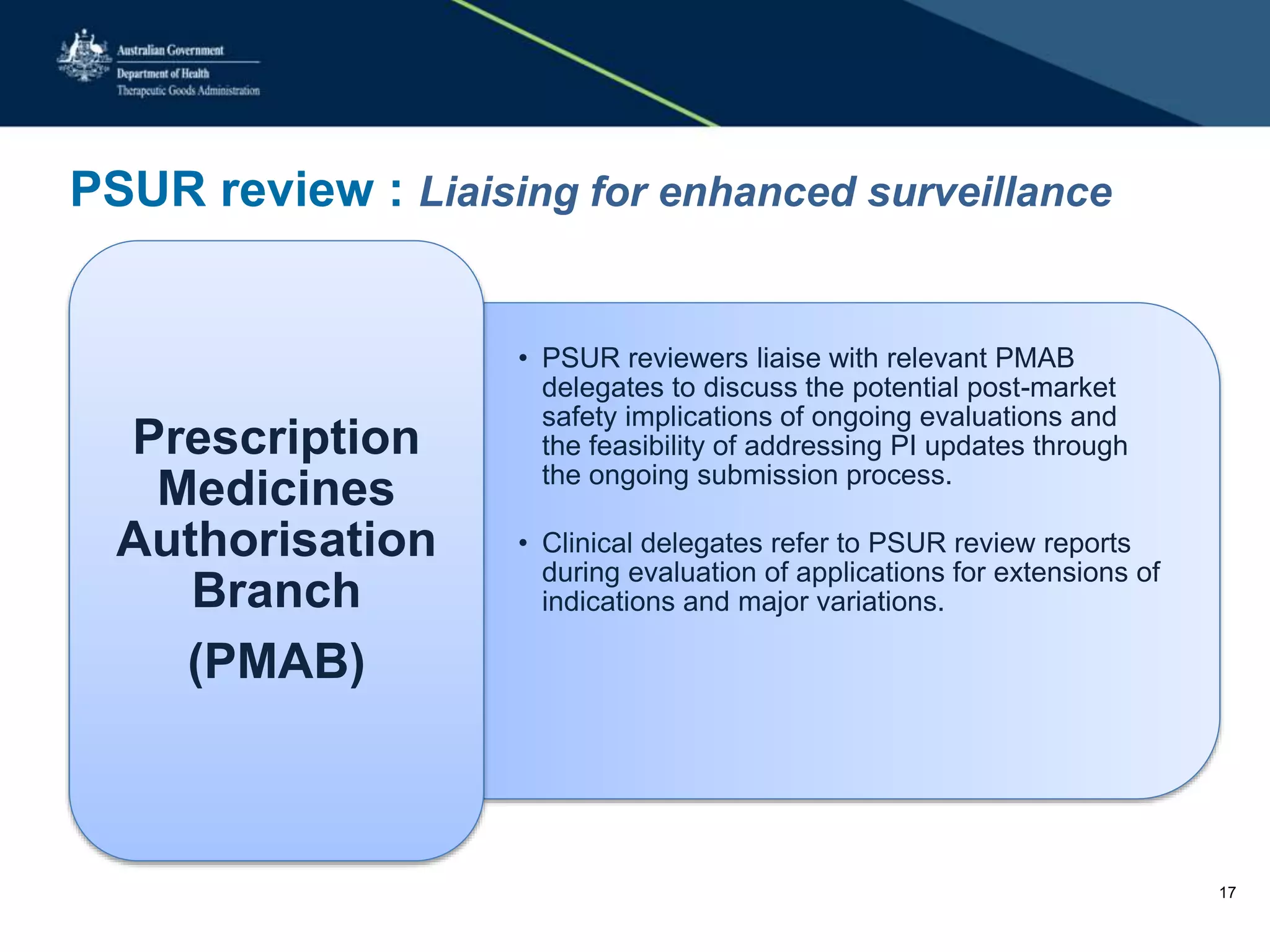
The TGA approach to reviewing PSURs (Periodic Safety Update Reports) focuses on evaluating new or emerging safety information to assess the benefit-risk balance of approved medicines. PSUR reviews are prioritized based on risk factors like a product's safety profile and therapeutic importance. If a safety issue is identified that warrants a label change, the PSUR reviewer will recommend an update to the sponsor. Requests may also be made for additional monitoring or analyses in future PSURs. PSUR reviewers collaborate with other TGA departments to enhance post-market vigilance and ensure safety signals are investigated. Sponsors can expect direct communication regarding recommendations from PSUR reviews and are encouraged to submit PSURs using the eCTD format.