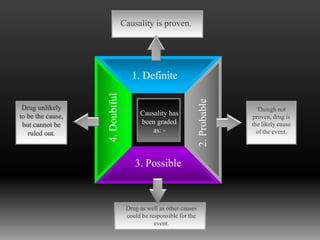
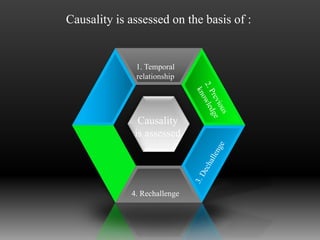
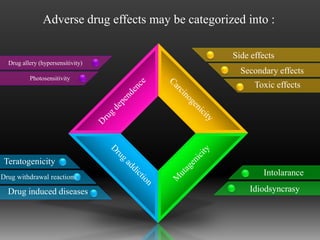
Pharmacovigilance is defined by the WHO as the science and activities related to detecting, assessing, understanding, and preventing adverse effects from medicines. Its main purpose is to reduce the risk of harm to patients from drug use. Pharmacovigilance involves post-marketing surveillance methods like voluntary reporting of adverse drug reactions, as well as disseminating data on ADRs to educate doctors and regulatory bodies.