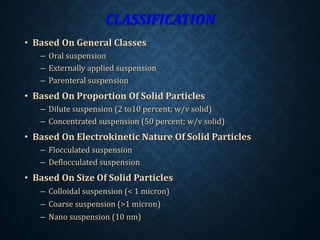
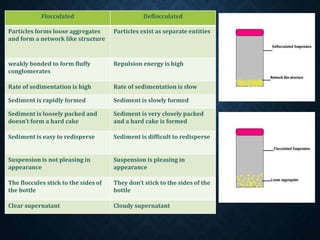
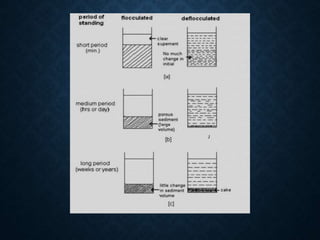
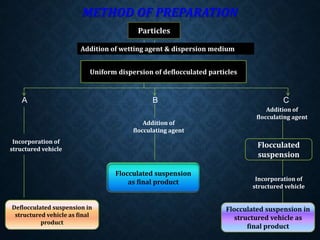
The document discusses pharmaceutical suspensions, describing them as coarse dispersions of insoluble materials in liquid mediums, primarily used for administering poorly soluble drugs. It outlines their advantages, such as improved chemical stability and bioavailability, as well as disadvantages like physical stability issues and formulation challenges. Various types, classifications, formulations, preparation methods, and application routes of suspensions are detailed, highlighting their importance in oral, topical, parenteral, and ophthalmic applications.