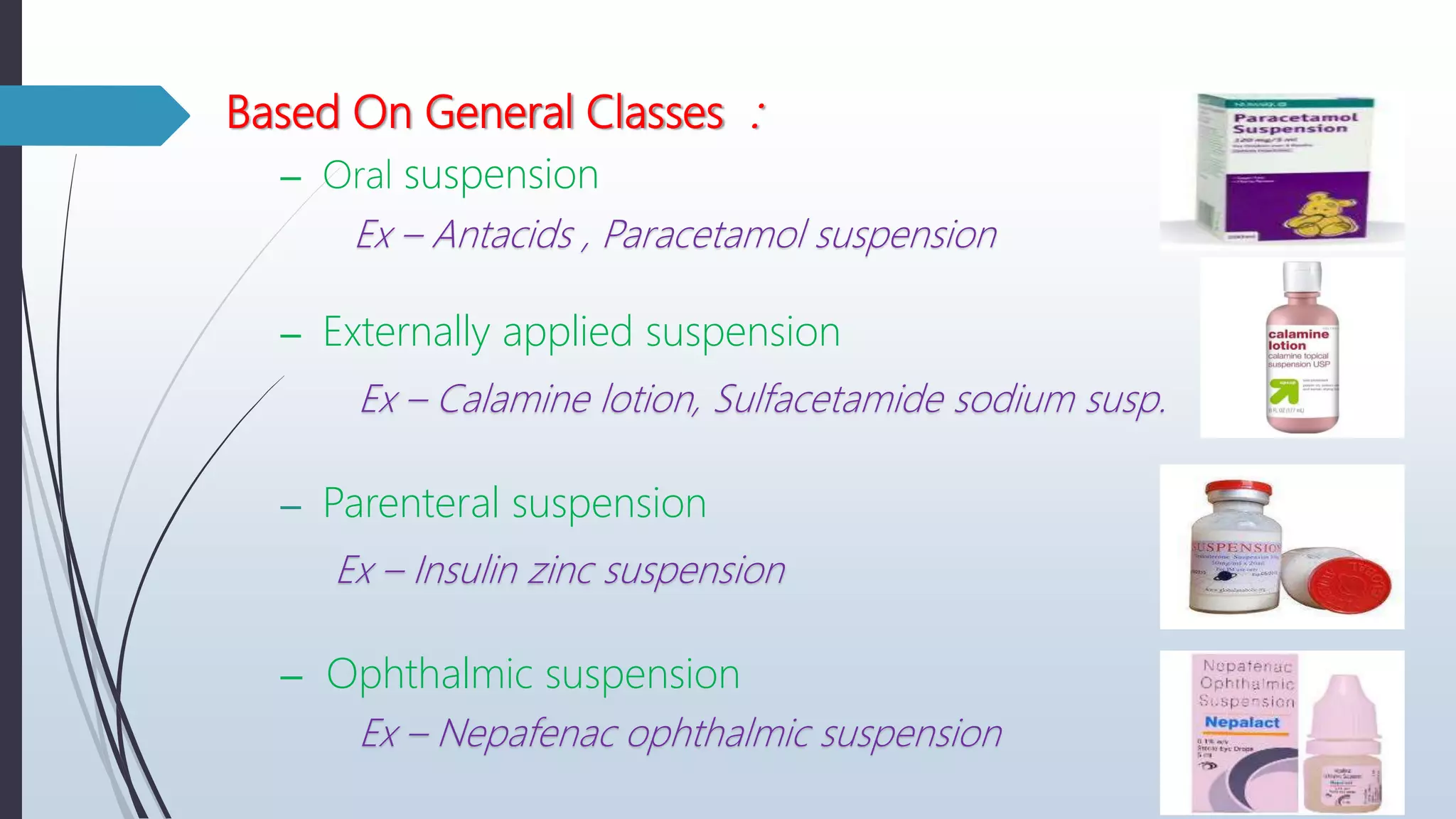
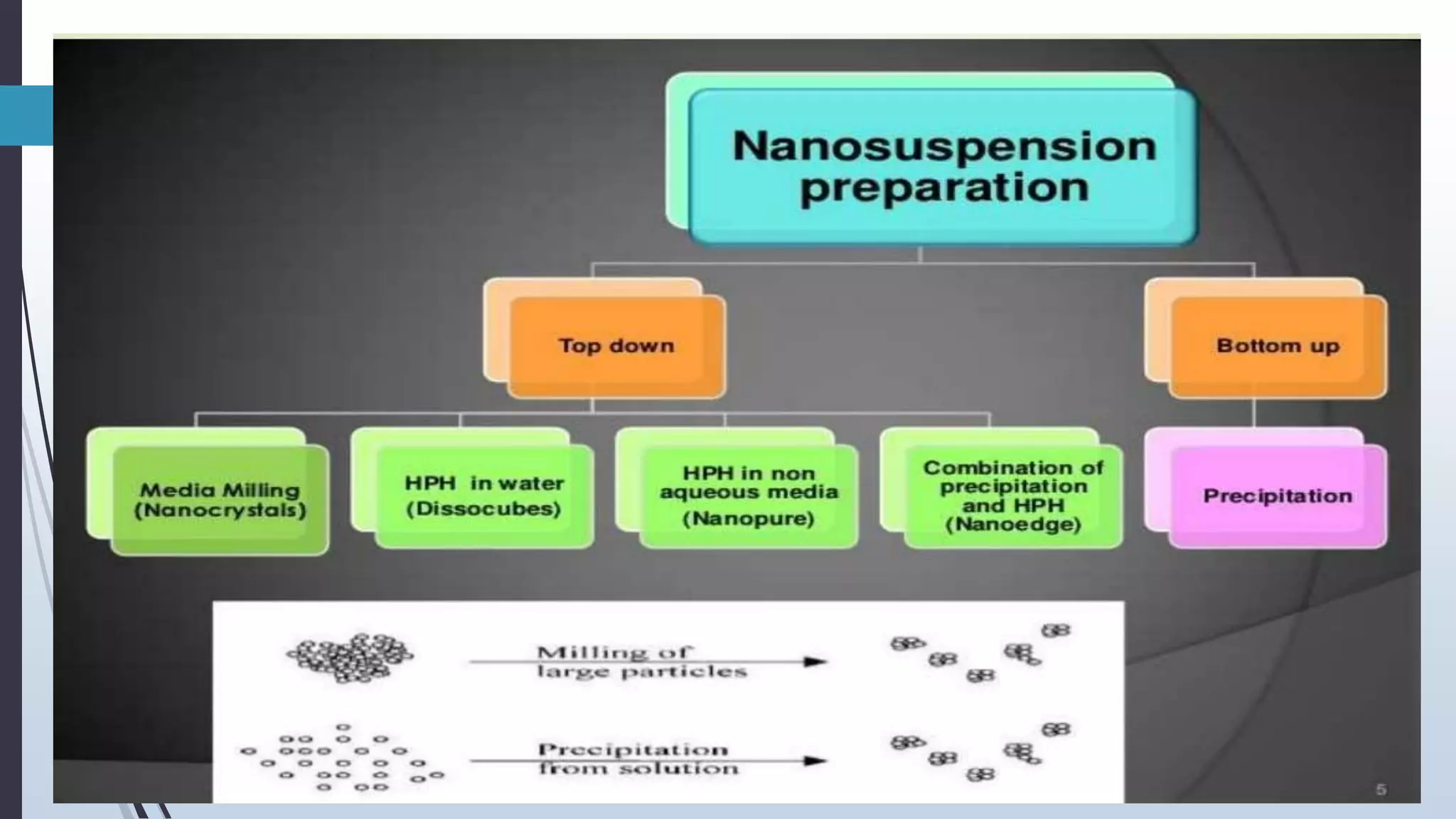
The document provides a comprehensive overview of pharmaceutical suspensions, including definitions, classifications, stability considerations, formulation, preparation, and quality control. It discusses the properties of suspensions, relevant theoretical concepts like sedimentation and Brownian movement, and advancements such as nano suspensions and taste-masked formulations. Key formulation components and methods for stabilizing suspensions are also outlined.