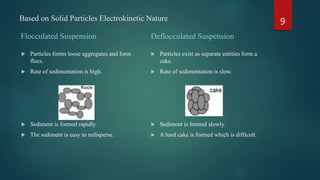
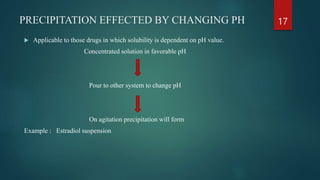
The document discusses pharmaceutical suspensions, defining them as biphasic coarse dispersions with an internal phase of insoluble solid particles dispersed in an external phase. It outlines the reasons for formulating suspensions, criteria for a good suspension, and discusses various types of suspensions based on administration routes and solid particle characteristics. Additionally, it covers the role of suspending agents, wetting agents, and preservatives, along with preparation methods and evaluation techniques.