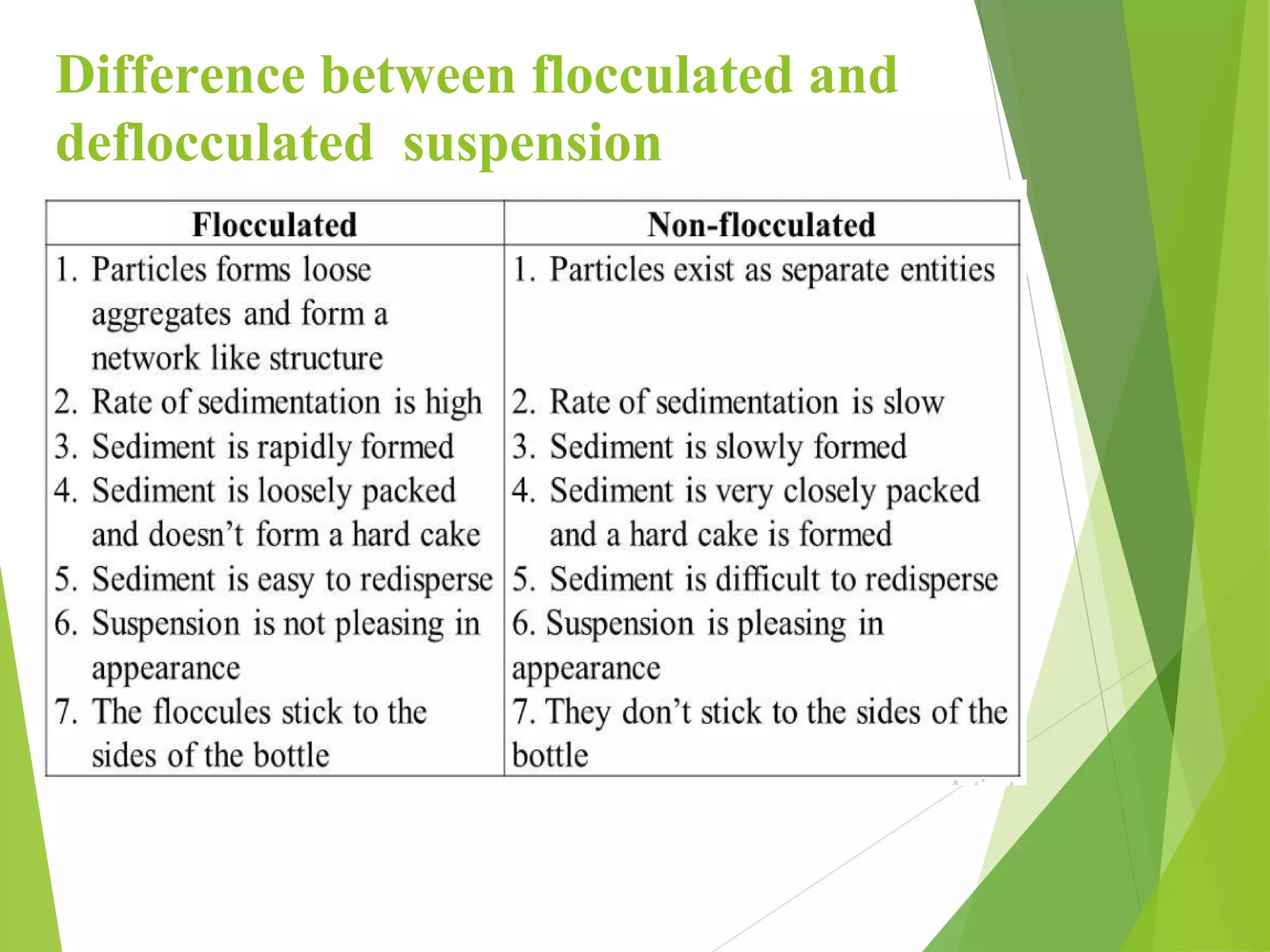
This document discusses pharmaceutical suspensions, defining them as disperse systems with insoluble drug particles uniformly distributed in a vehicle, typically aqueous. It covers classifications, types of suspending agents, and reasons for formulating drugs as suspensions, emphasizing their use for poorly soluble drugs and masking unpleasant tastes. The document also highlights the advantages of suspensions, including improved stability and bioavailability compared to other dosage forms.