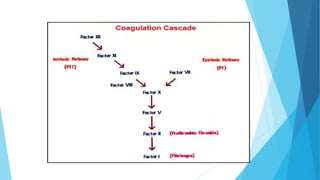
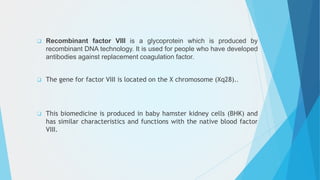
The document discusses the development of a biopharmaceutical for hemophilia A, focusing on antihemophilic factor VIII, which is critical for blood clotting. It describes the mechanisms of hemophilia, incidence rates, management strategies, and advancements in recombinant coagulation factors, including the product Lyoclot. The production and formulation details of recombinant factor VIII are provided, emphasizing its storage and administration requirements.