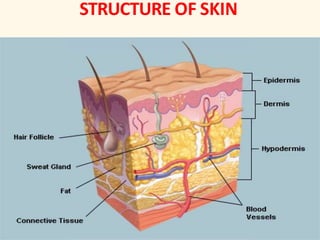
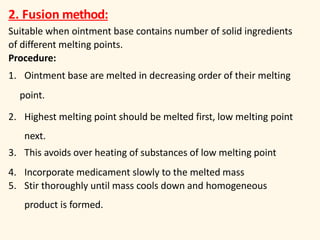
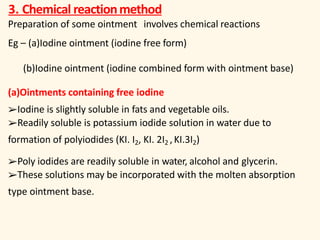
The document discusses semisolid dosage forms such as ointments, describing their uses, types of ointment bases, advantages and disadvantages of ointments, preparation methods, and factors to consider when selecting vehicle bases for dermatological formulations. Ointments can be used topically to treat skin conditions and provide local or systemic drug effects depending on the formulation. Selection of the appropriate ointment base depends on factors like absorption, skin compatibility, stability, and consistency.