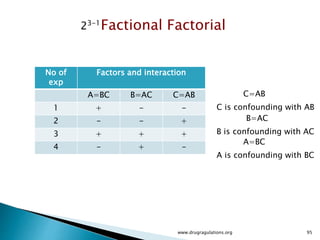
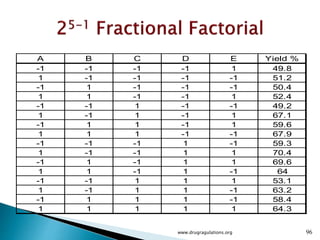
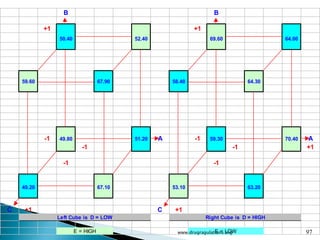
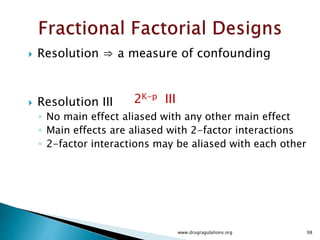
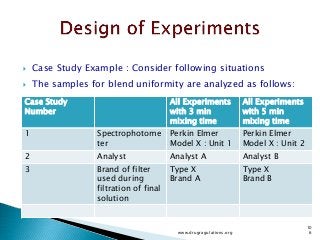
This document provides information about a case study on the blending process for a pharmaceutical formulation. It includes:
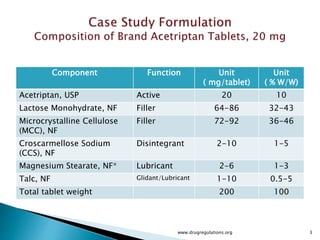
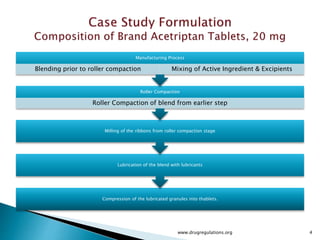
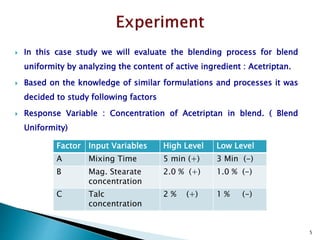
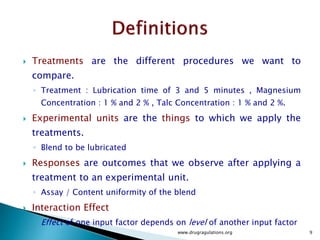
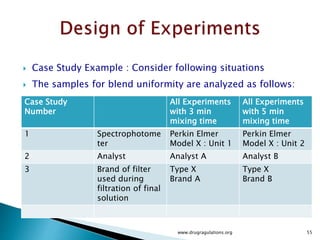
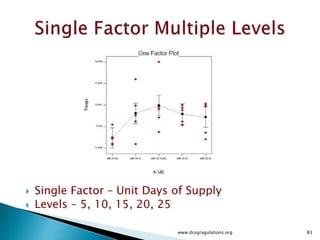
1) Details of the formulation and factors being studied (mixing time, magnesium stearate concentration, and talc concentration) to evaluate blend uniformity.
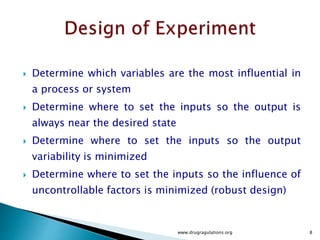
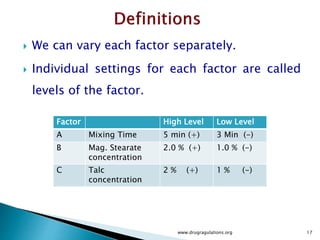
2) Descriptions of key concepts for experimental design including treatments, experimental units, responses, and interactions.
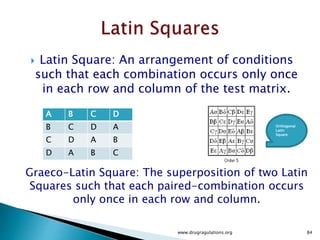
3) Discussion of blocking as a technique to reduce nuisance factors like different batches of active ingredients being studied.



















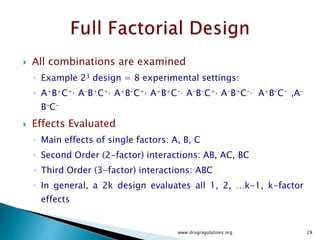

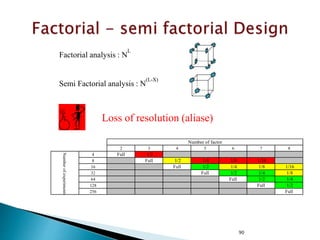
![30
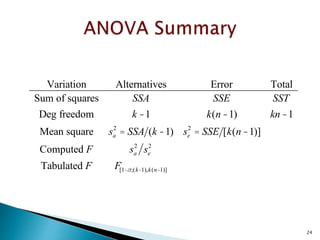
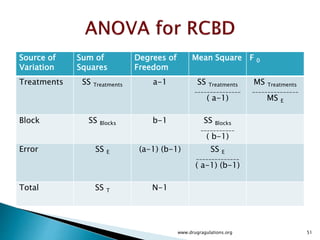
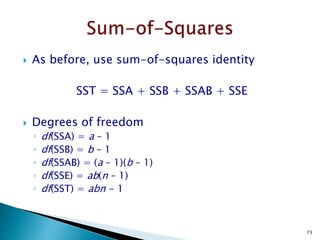
Variation Alternatives Error Total
Sum of squares SSA SSE SST
Deg freedom k -1 k(n -1) kn -1
Mean square sa
2
= SSA (k -1) se
2
= SSE [k(n -1)]
Computed F sa
2
se
2
Tabulated F F[1-a;(k-1),k(n-1)]](https://image.slidesharecdn.com/designofexperimetsforbegginers-150814095059-lva1-app6892/85/Pharmaceutical-Design-of-Experiments-for-Beginners-30-320.jpg)



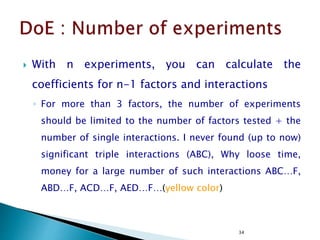
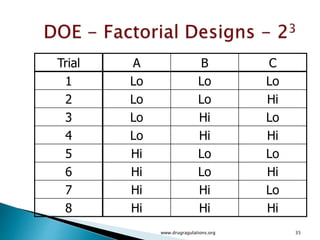











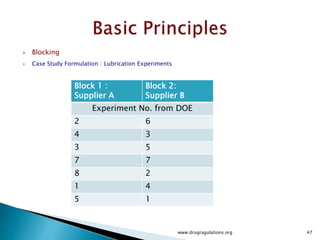



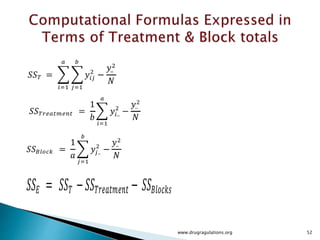
















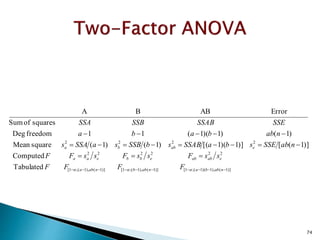


























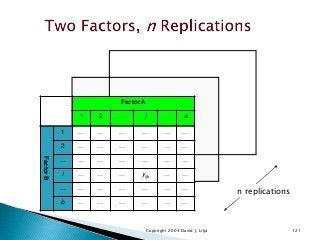
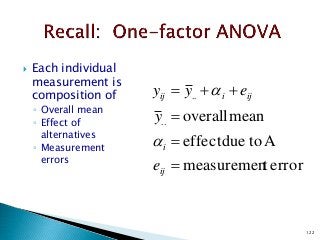
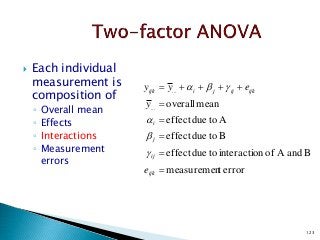
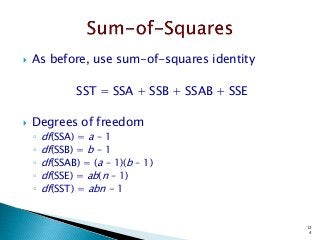
![12
5
)]1(),1)(1(;1[)]1(),1(;1[)]1(),1(;1[
222222
2222
Tabulated
Computed
)]1([)]1)(1[()1()1(squareMean
)1()1)(1(11freedomDeg
squaresofSum
ErrorABBA
nabbanabbnaba
eababebbeaa
eabba
FFFF
ssFssFssFF
nabSSEsbaSSABsbSSBsaSSAs
nabbaba
SSESSABSSBSSA
](https://image.slidesharecdn.com/designofexperimetsforbegginers-150814095059-lva1-app6892/85/Pharmaceutical-Design-of-Experiments-for-Beginners-125-320.jpg)




