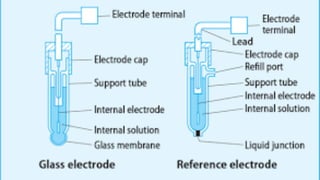
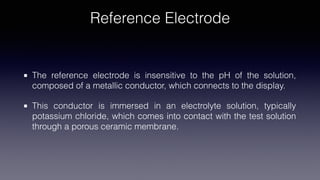
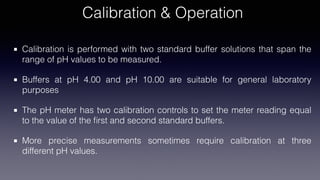
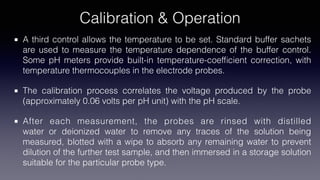
pH meters measure the hydrogen-ion concentration in solutions by determining the voltage difference between a pH electrode and a reference electrode immersed in the solution. The pH electrode, usually made of glass, detects the hydrogen-ion concentration in the solution, while the reference electrode provides a point of reference for the electrical potential. pH meters are calibrated using standard buffer solutions and can then be used to precisely measure pH levels, with applications across industries like chemistry, agriculture, food production, and healthcare.





![Types of pH meters
Benchtop pH meters are often used in laboratories and are used to
measure samples which are brought to the pH meter for analysis.
Portable, or
fi
eld pH meters, are handheld pH meters that are used to take
the pH of a sample in a
fi
eld or production site.[19]
In situ pH meters or pH analyzers, are used to measure pH continuously in
a process which could either stand-alone, or be connected to a higher-
level control information system for process control](https://image.slidesharecdn.com/phmeter-230228042416-003114f7/85/pH-meter-pdf-10-320.jpg)
