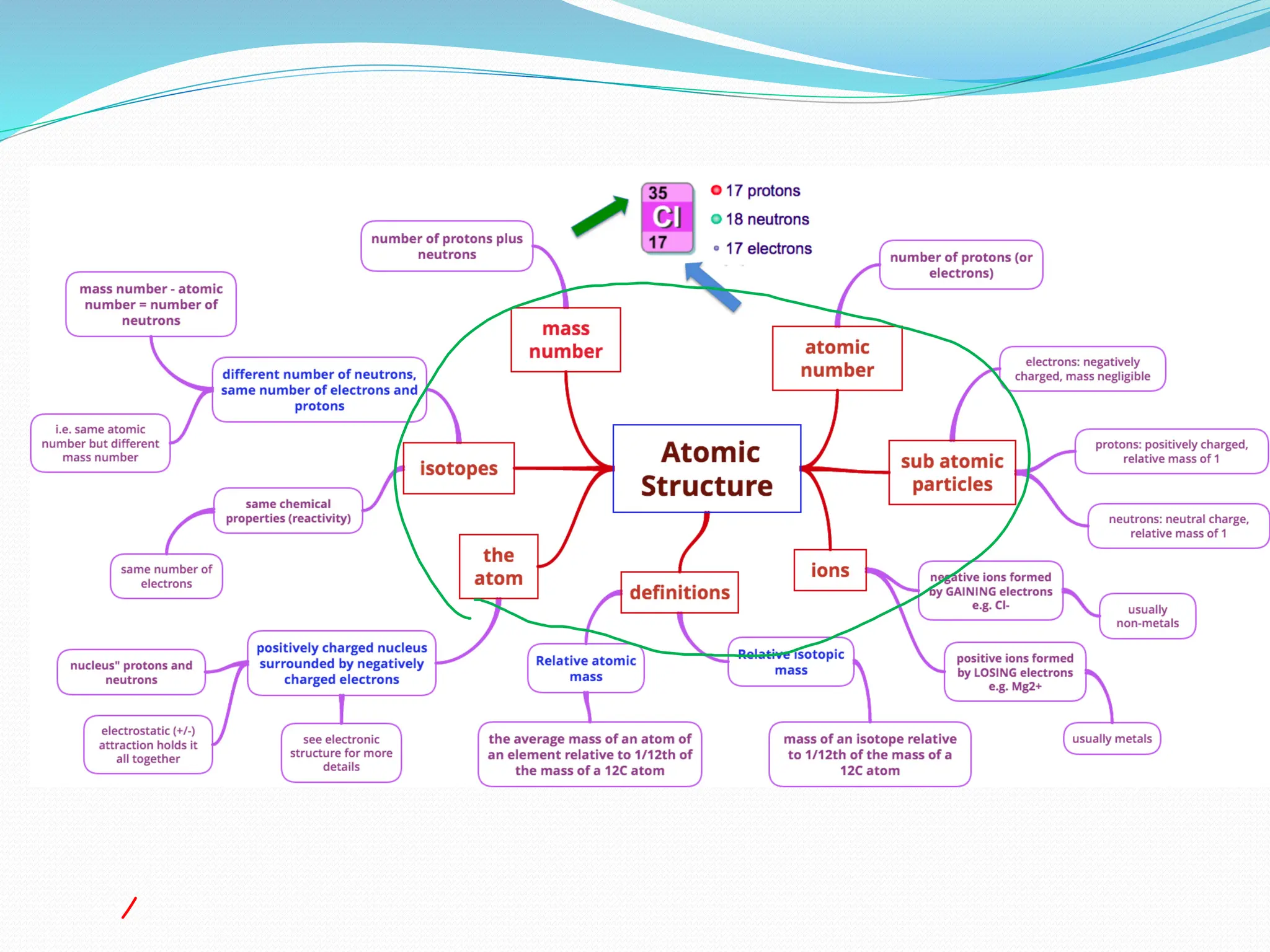
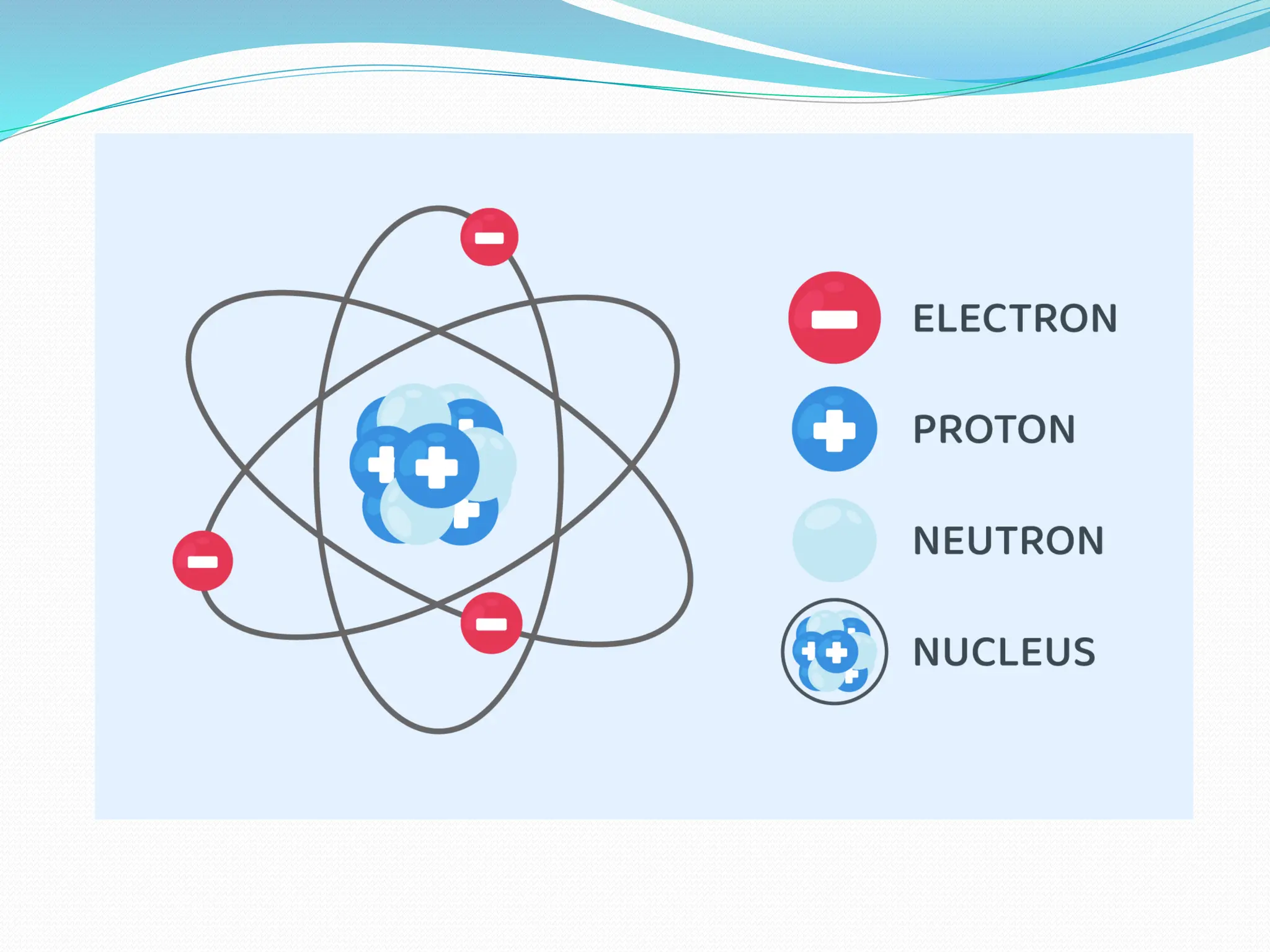
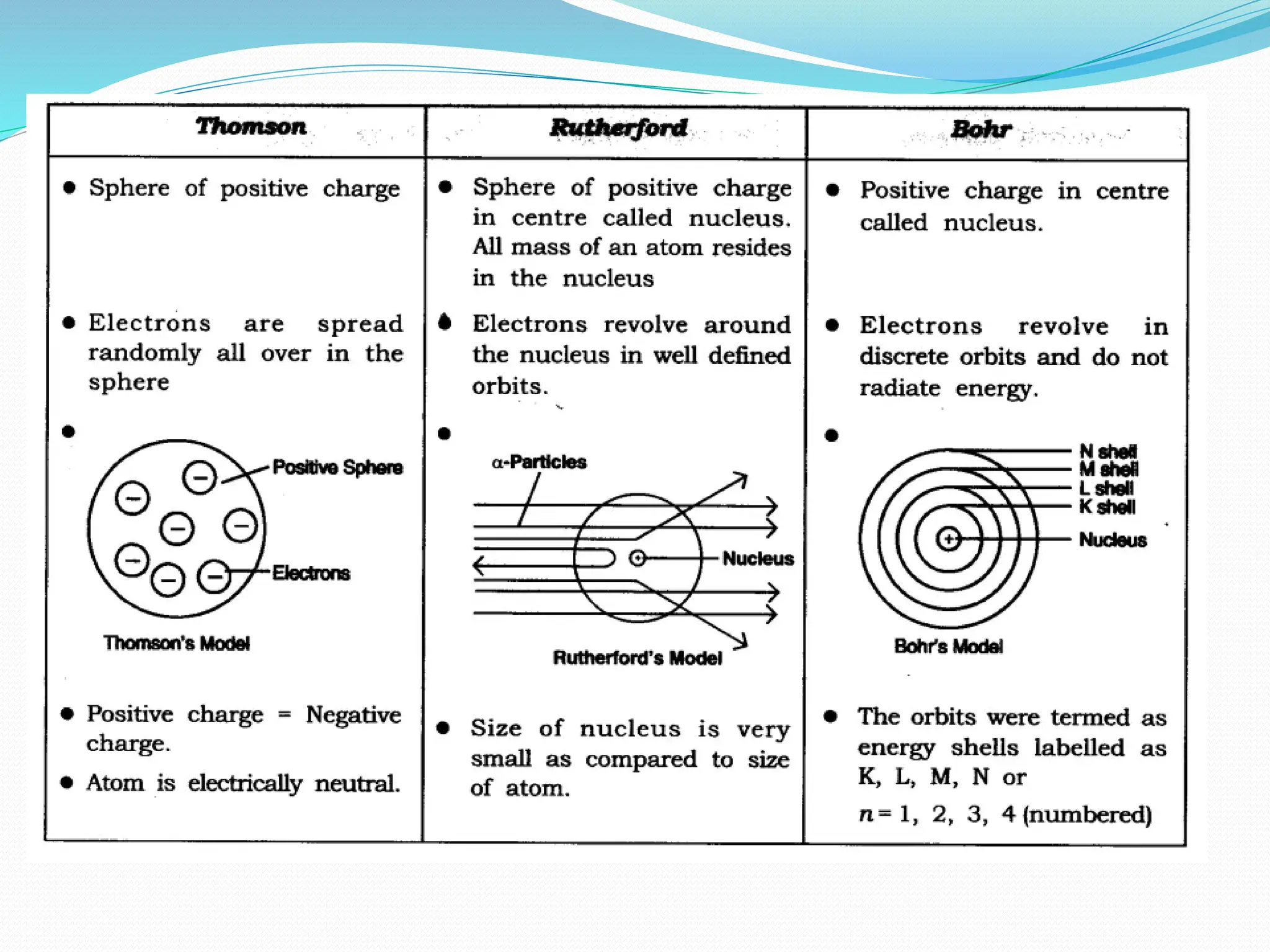
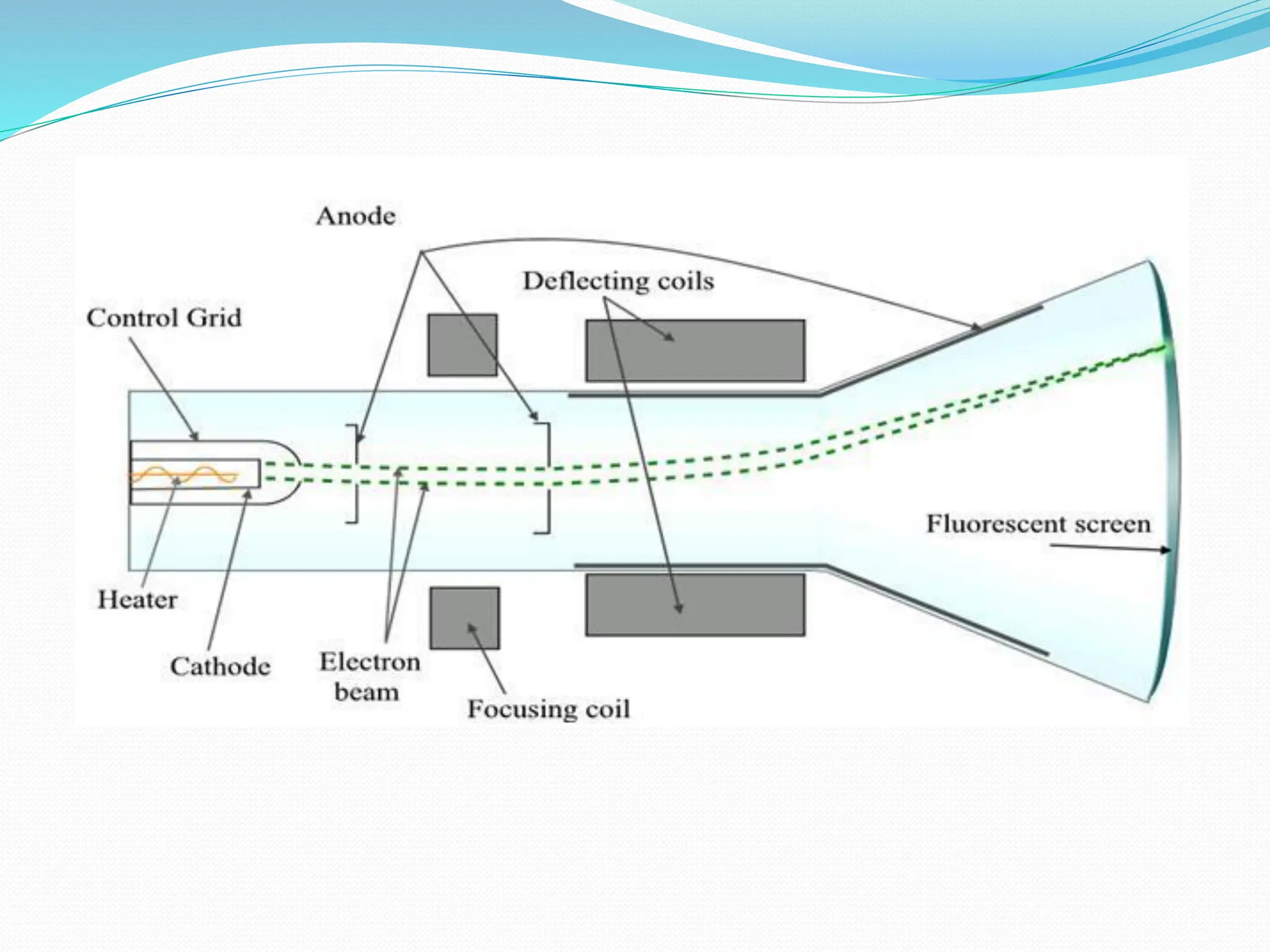
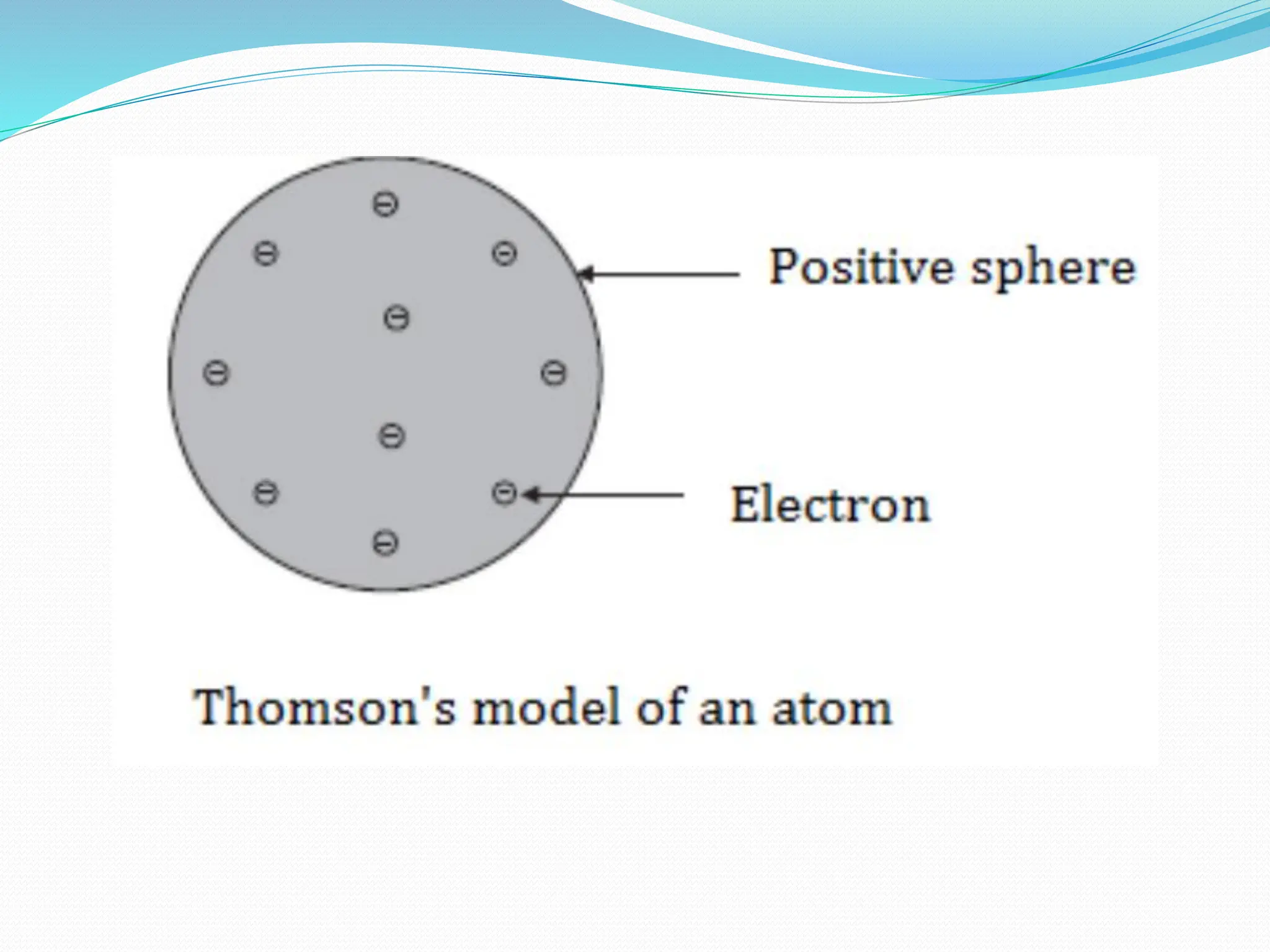
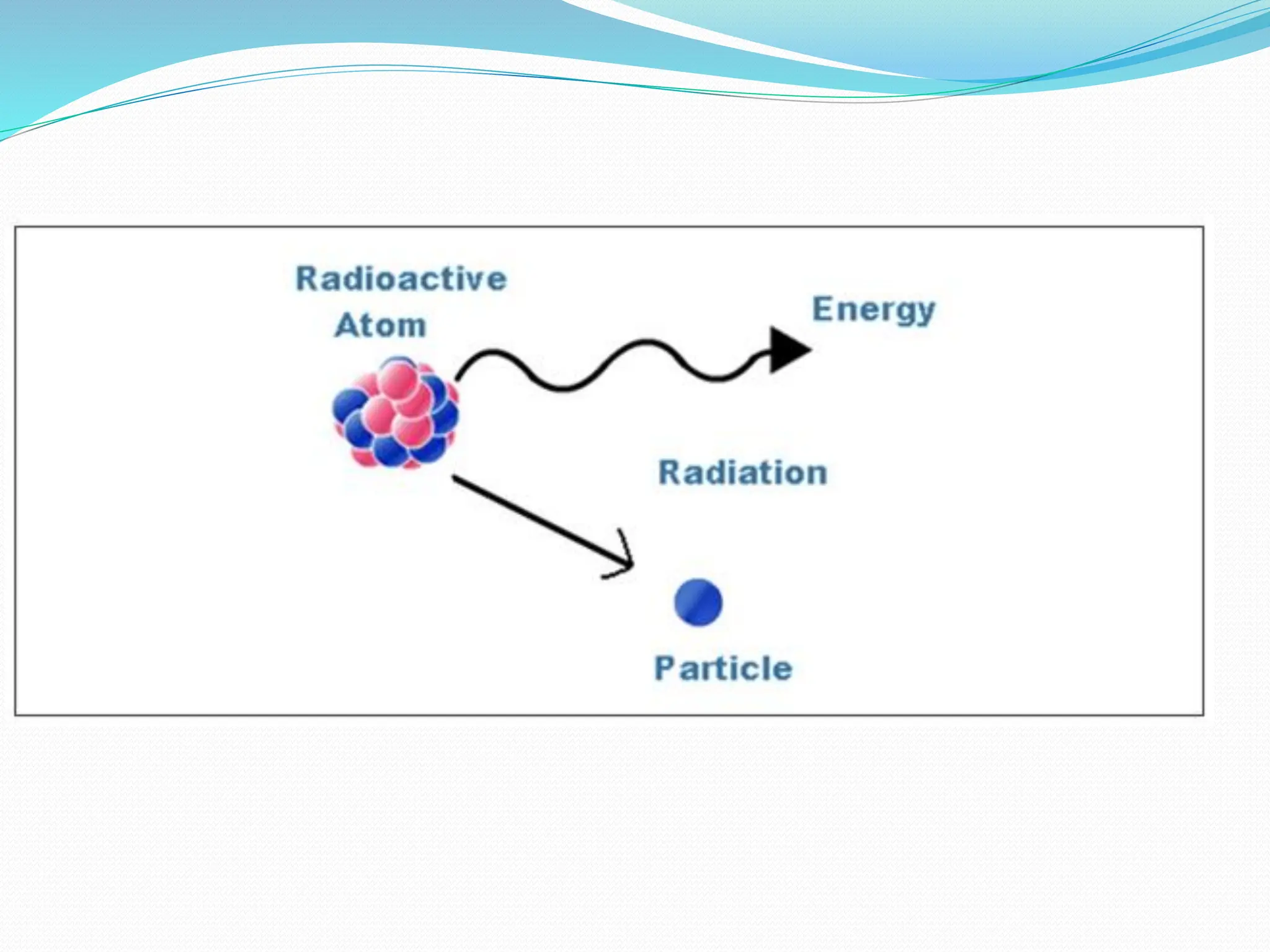
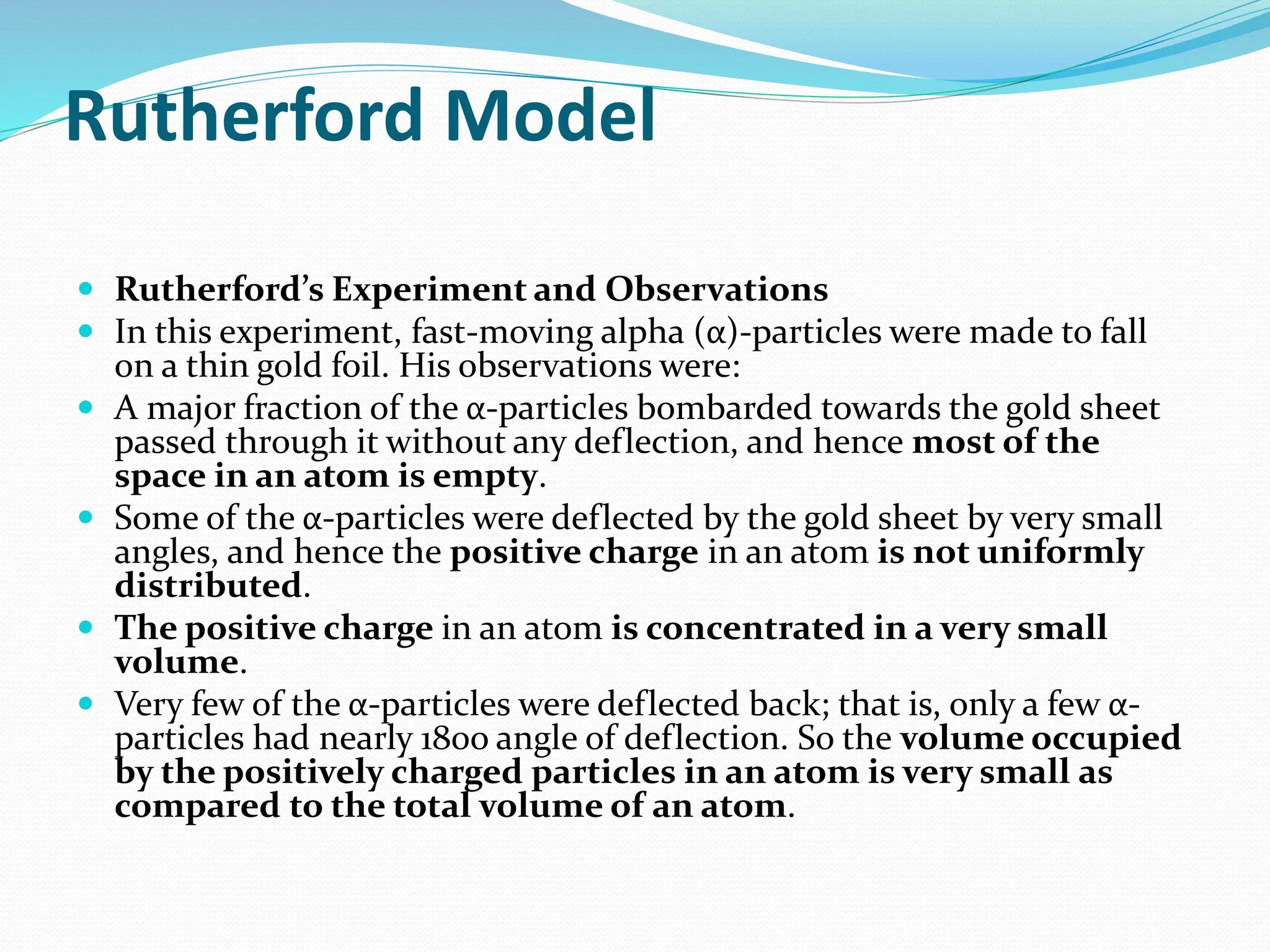
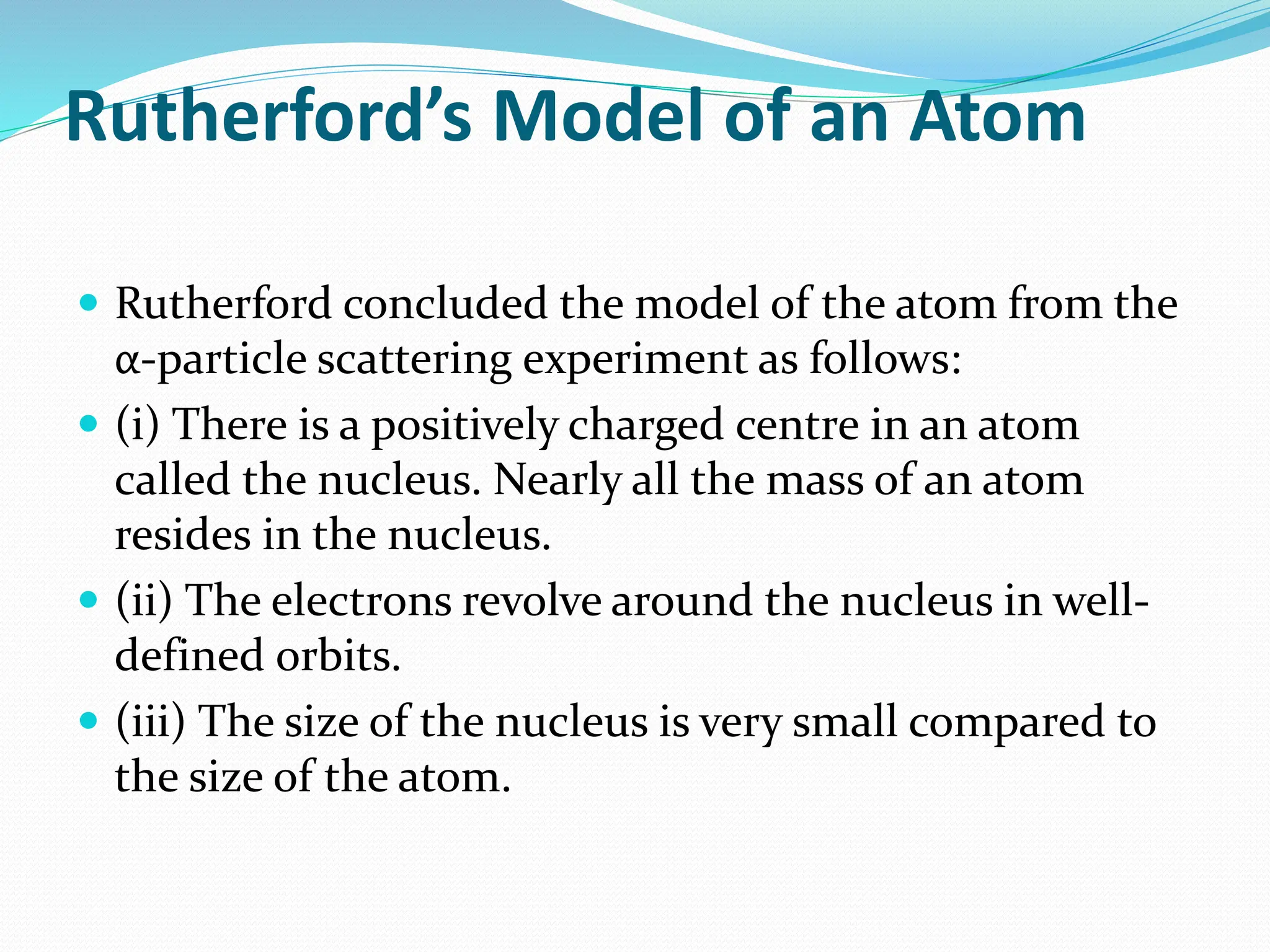
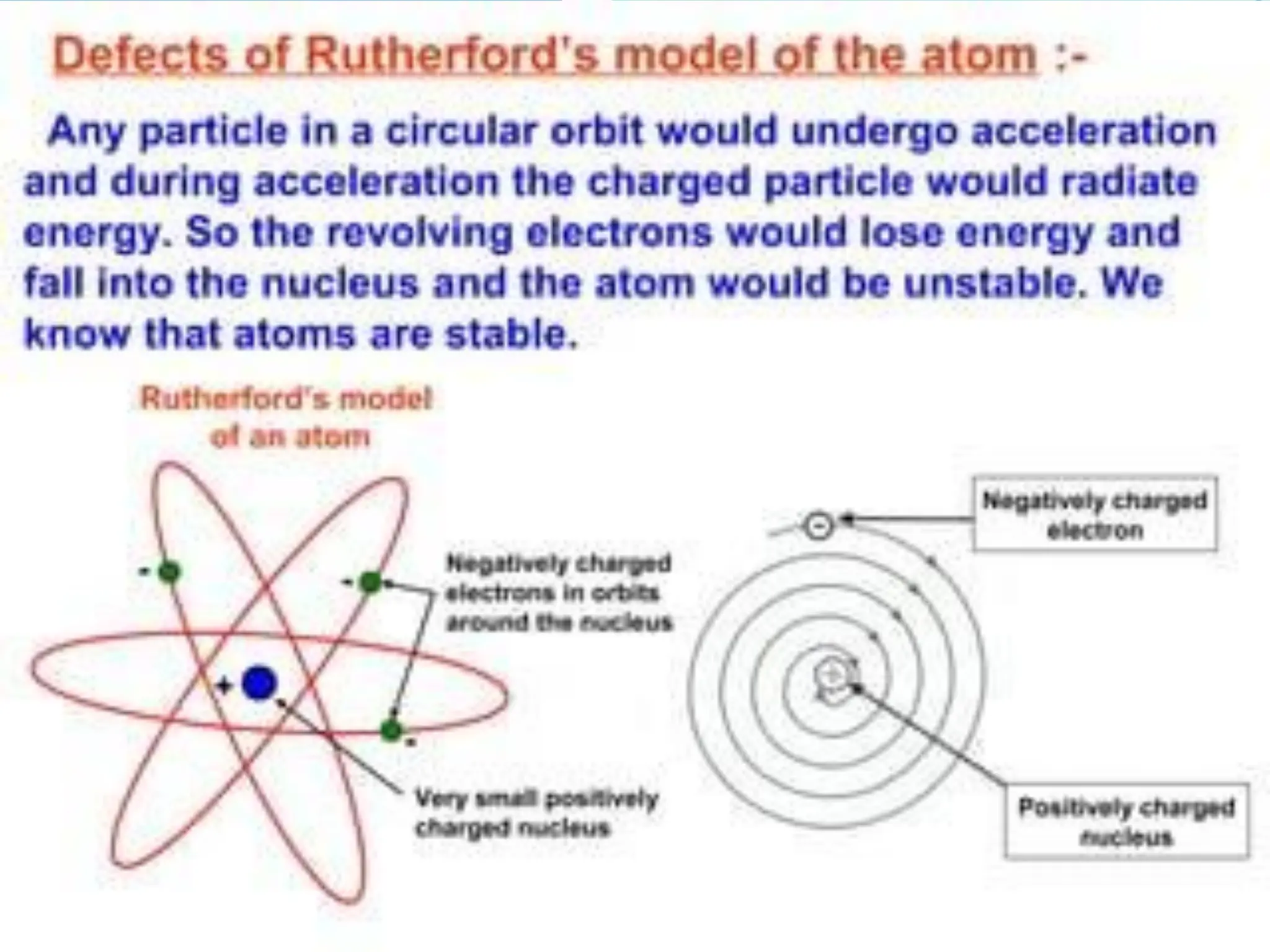
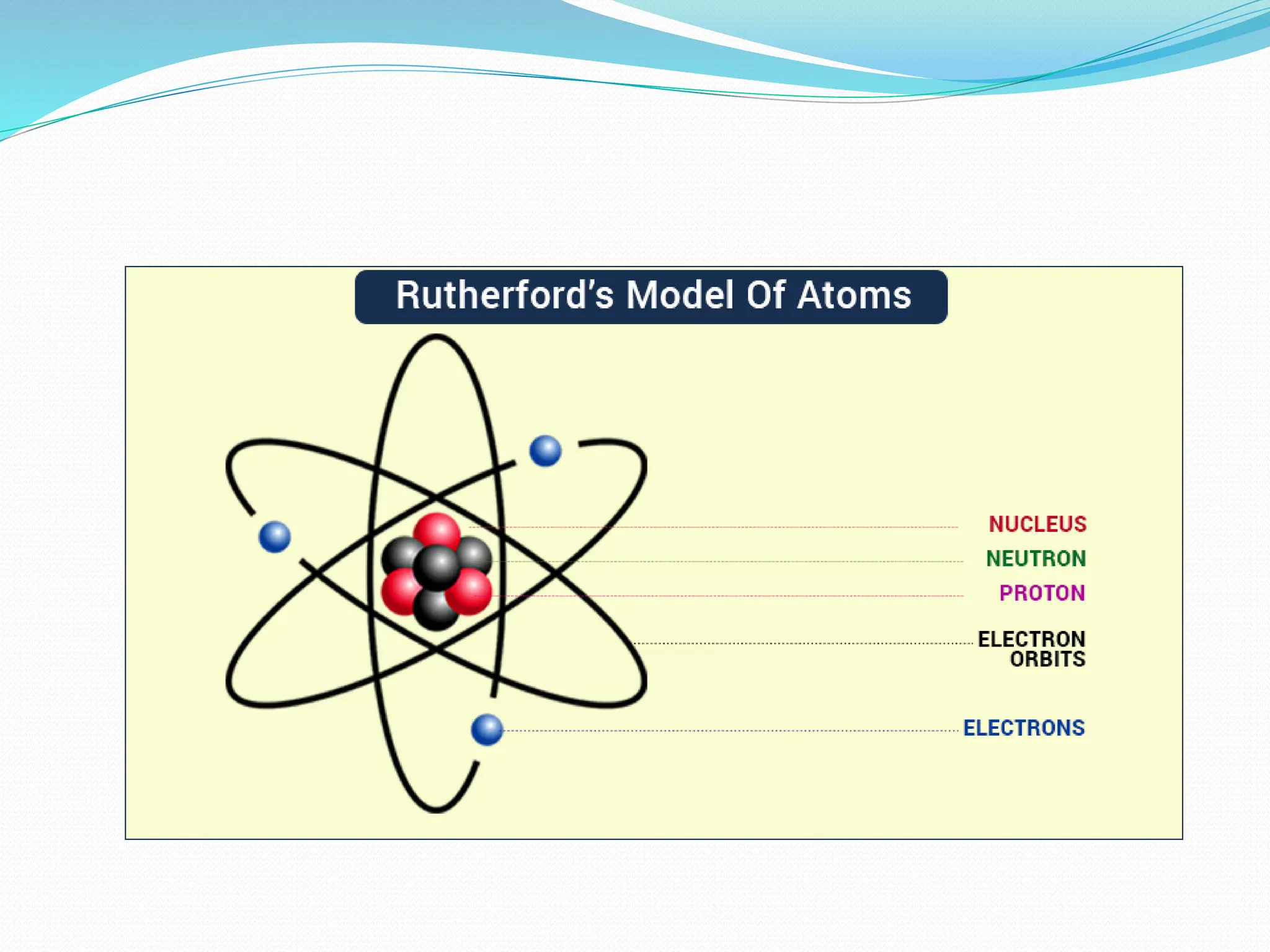
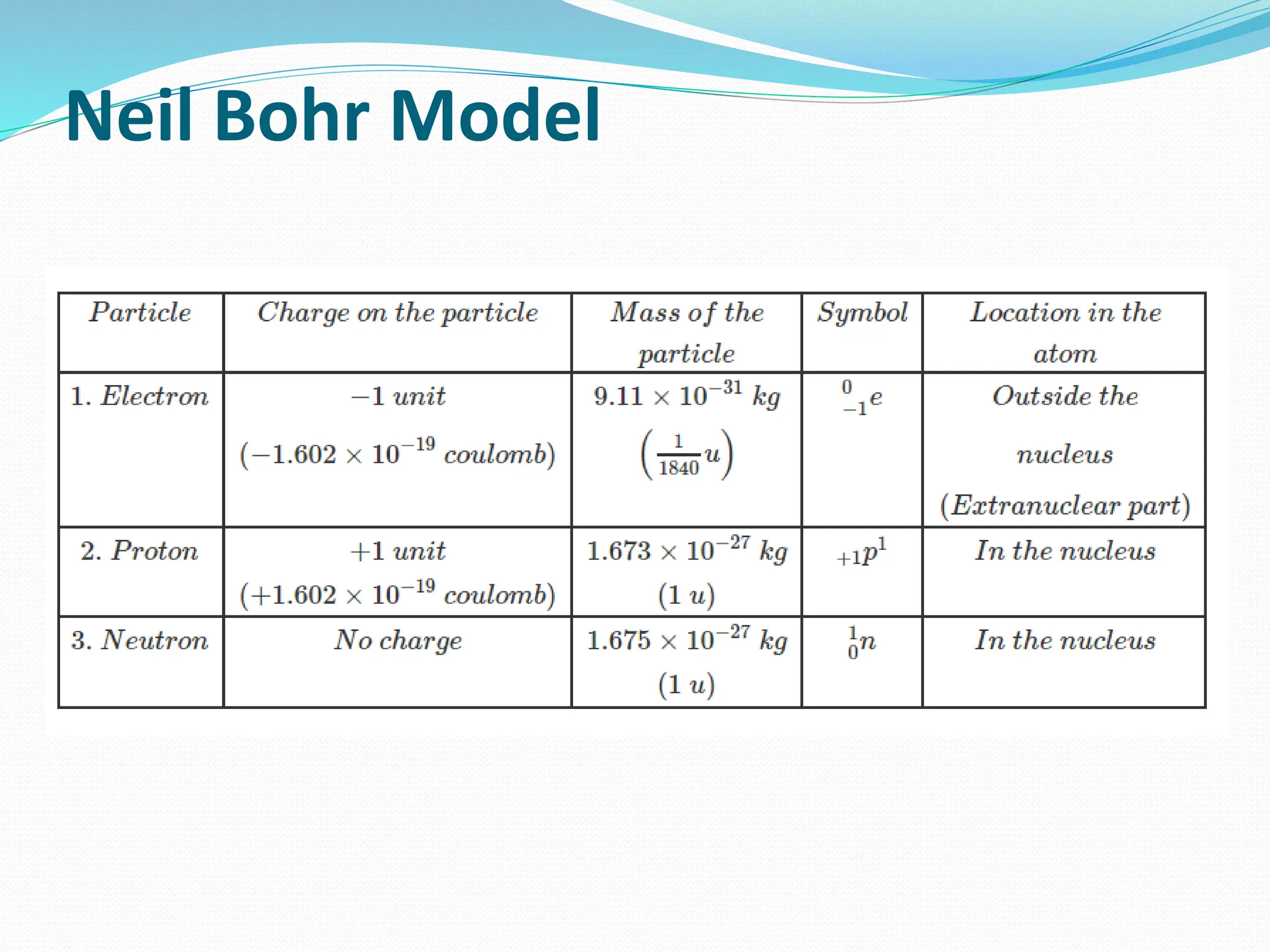
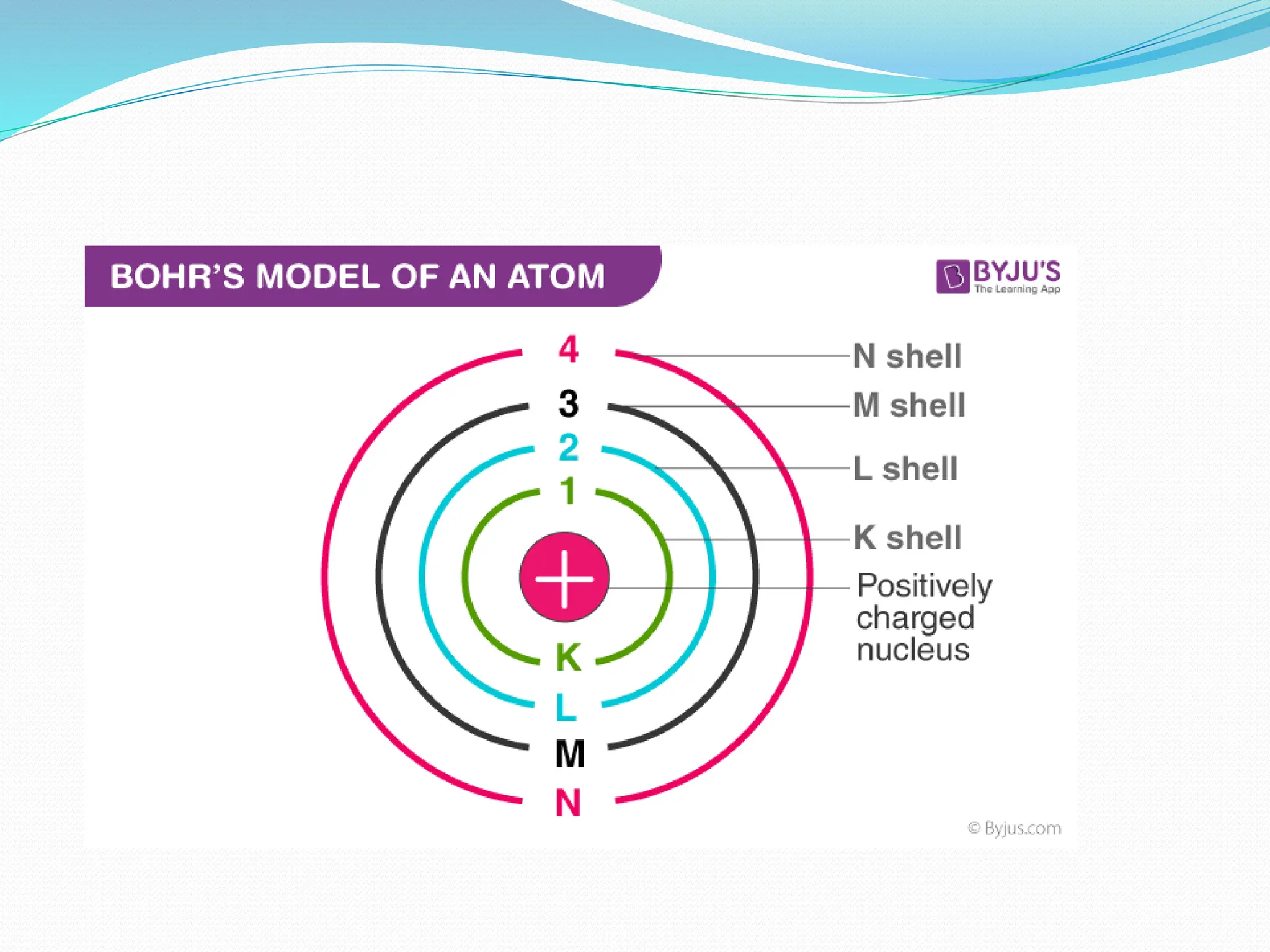
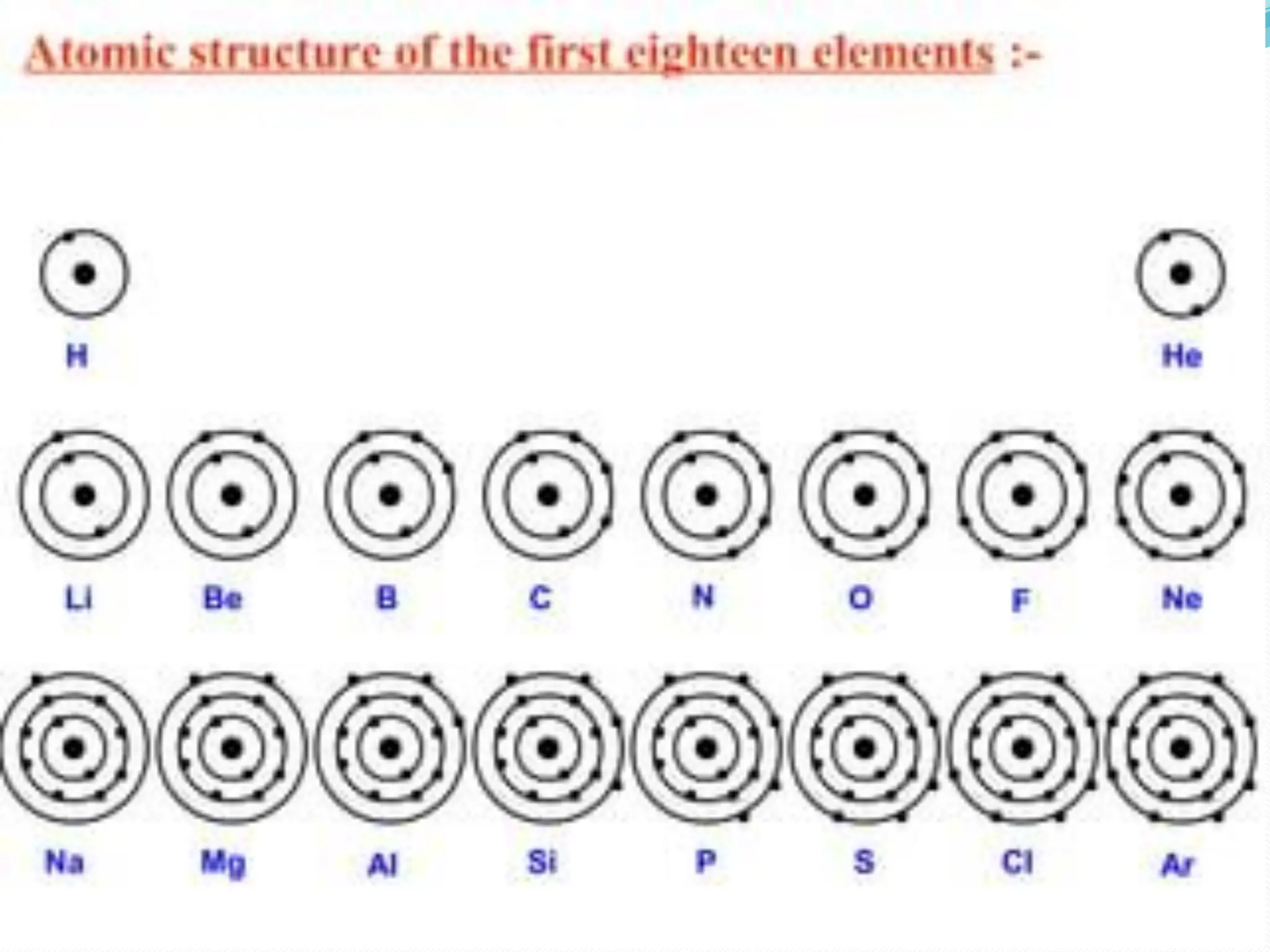
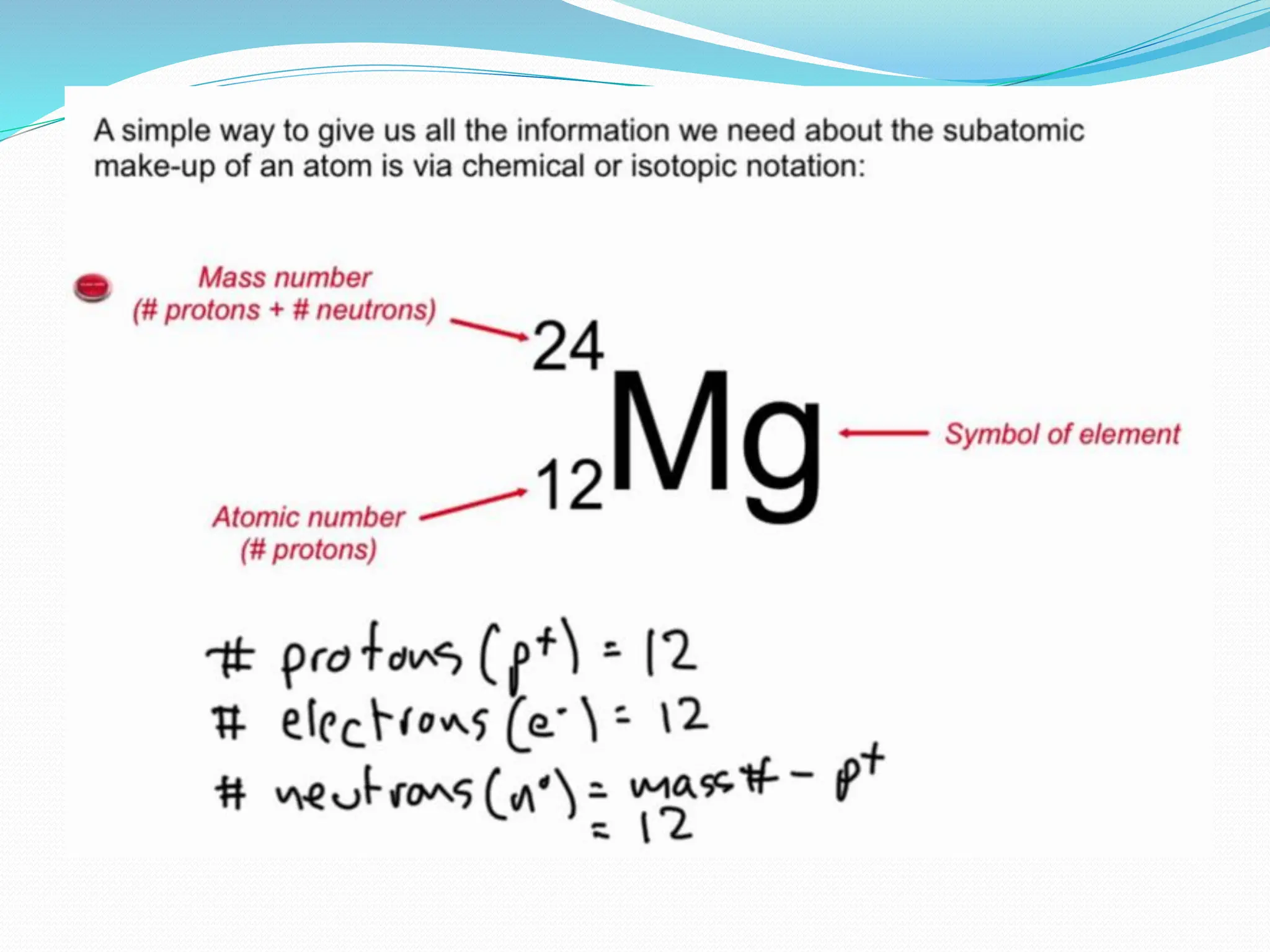
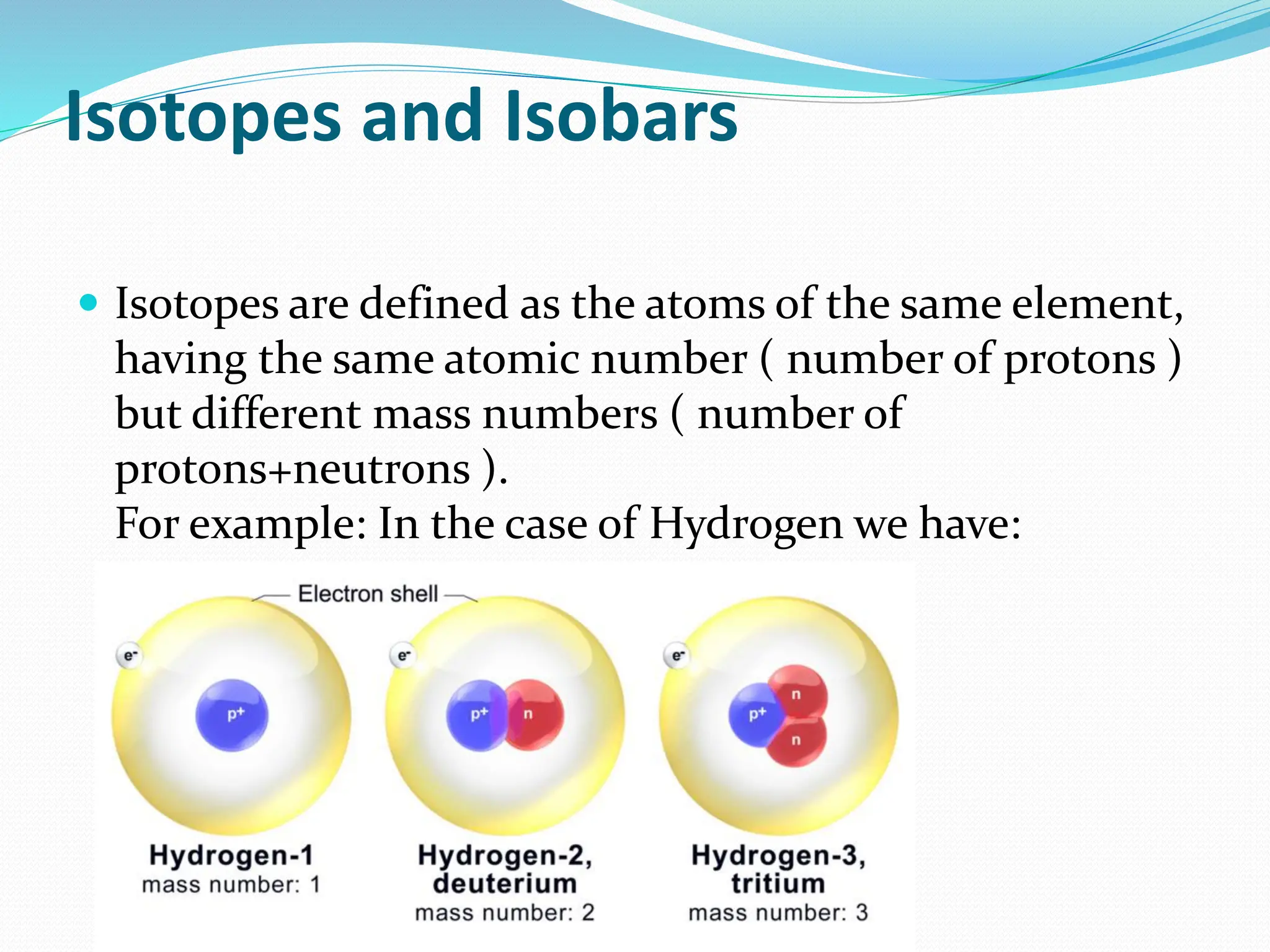
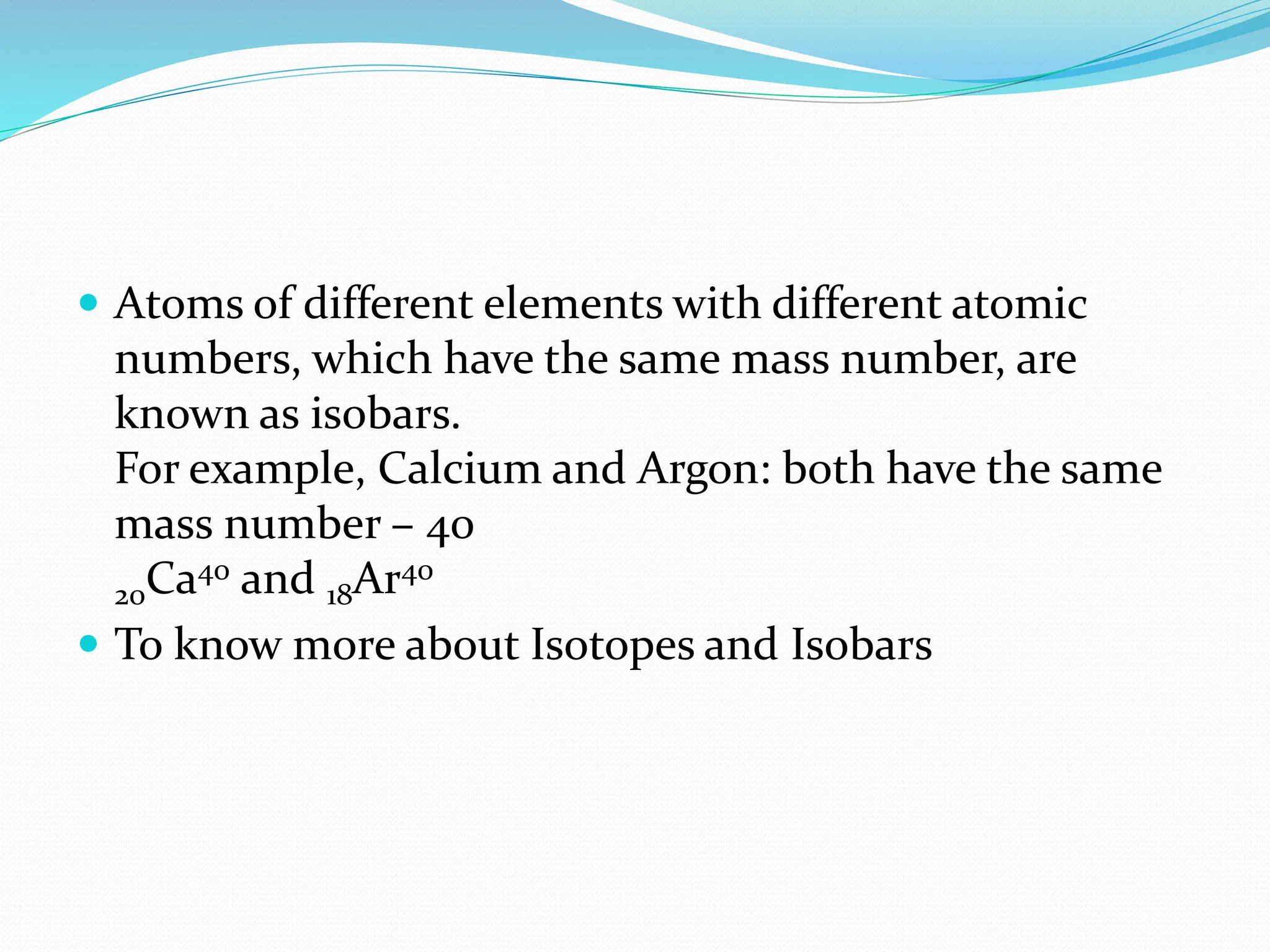
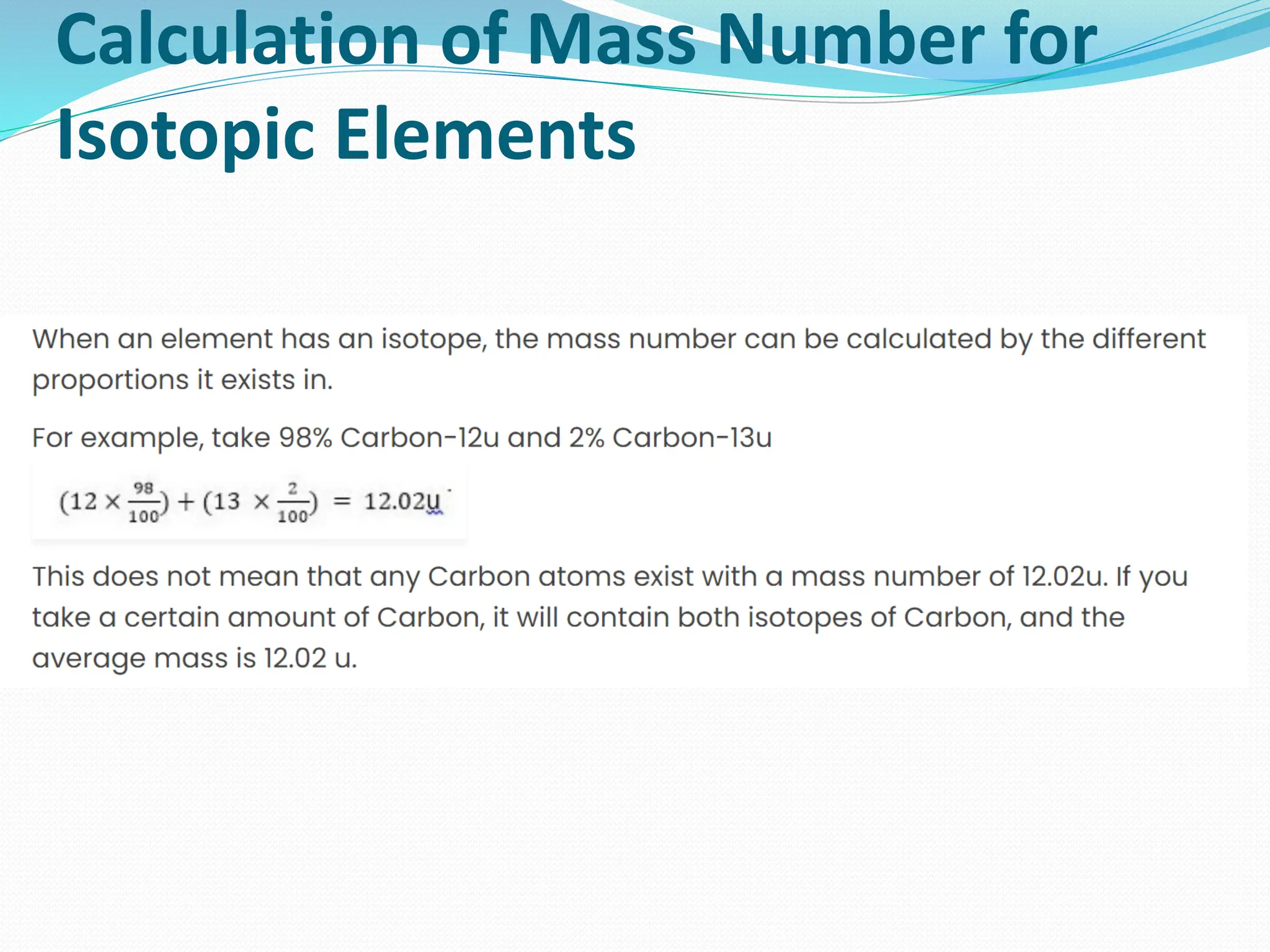
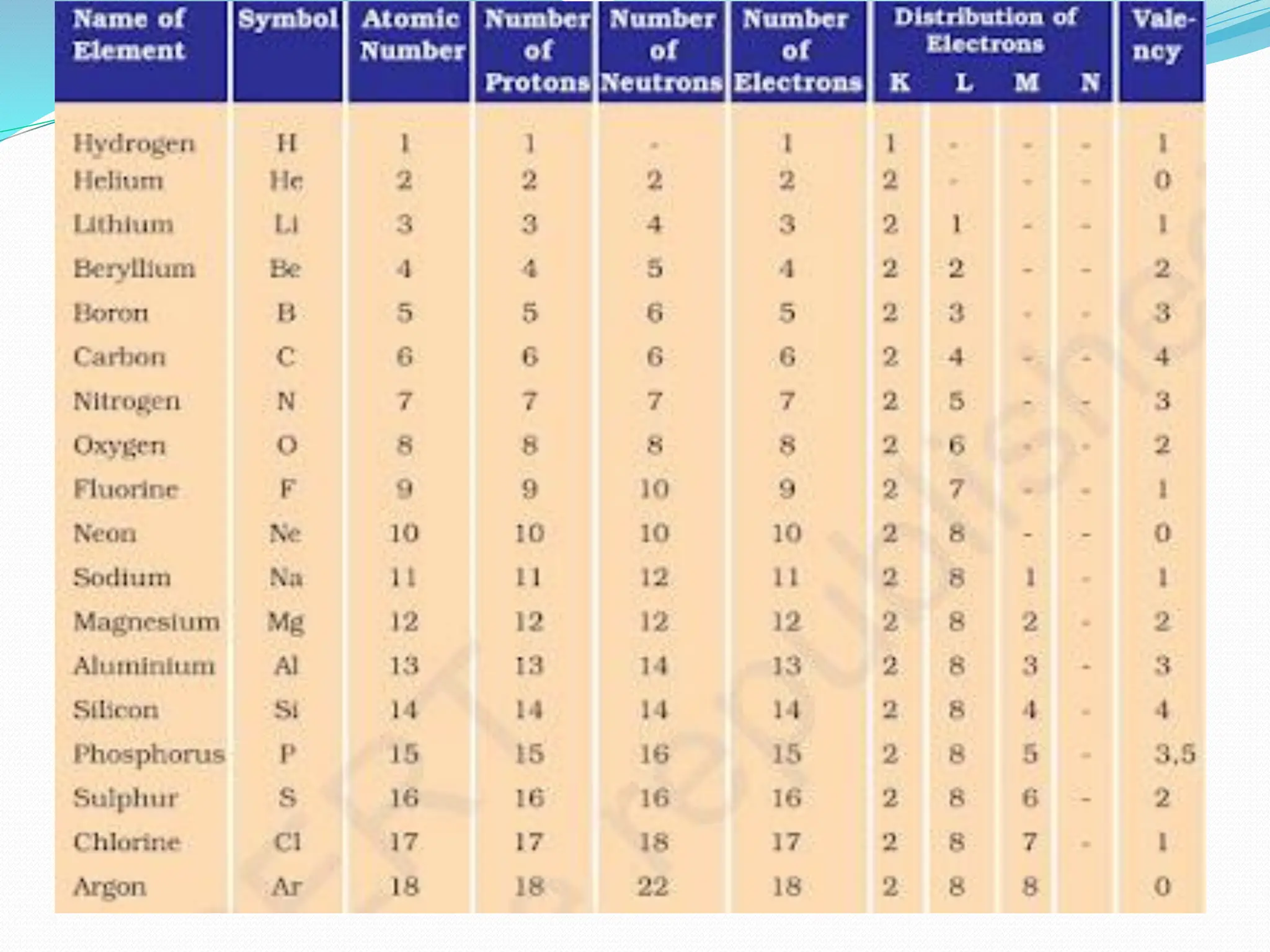
The document provides information on the structure of atoms, including key experiments and models that helped reveal the internal structure of atoms. It discusses J.J. Thomson's cathode ray experiment that discovered electrons, Rutherford's alpha particle scattering experiment that showed atoms have a small, dense nucleus, and Bohr's model of electron orbits around the nucleus. It also covers topics like isotopes, mass number, atomic number, electron configuration, and valency.