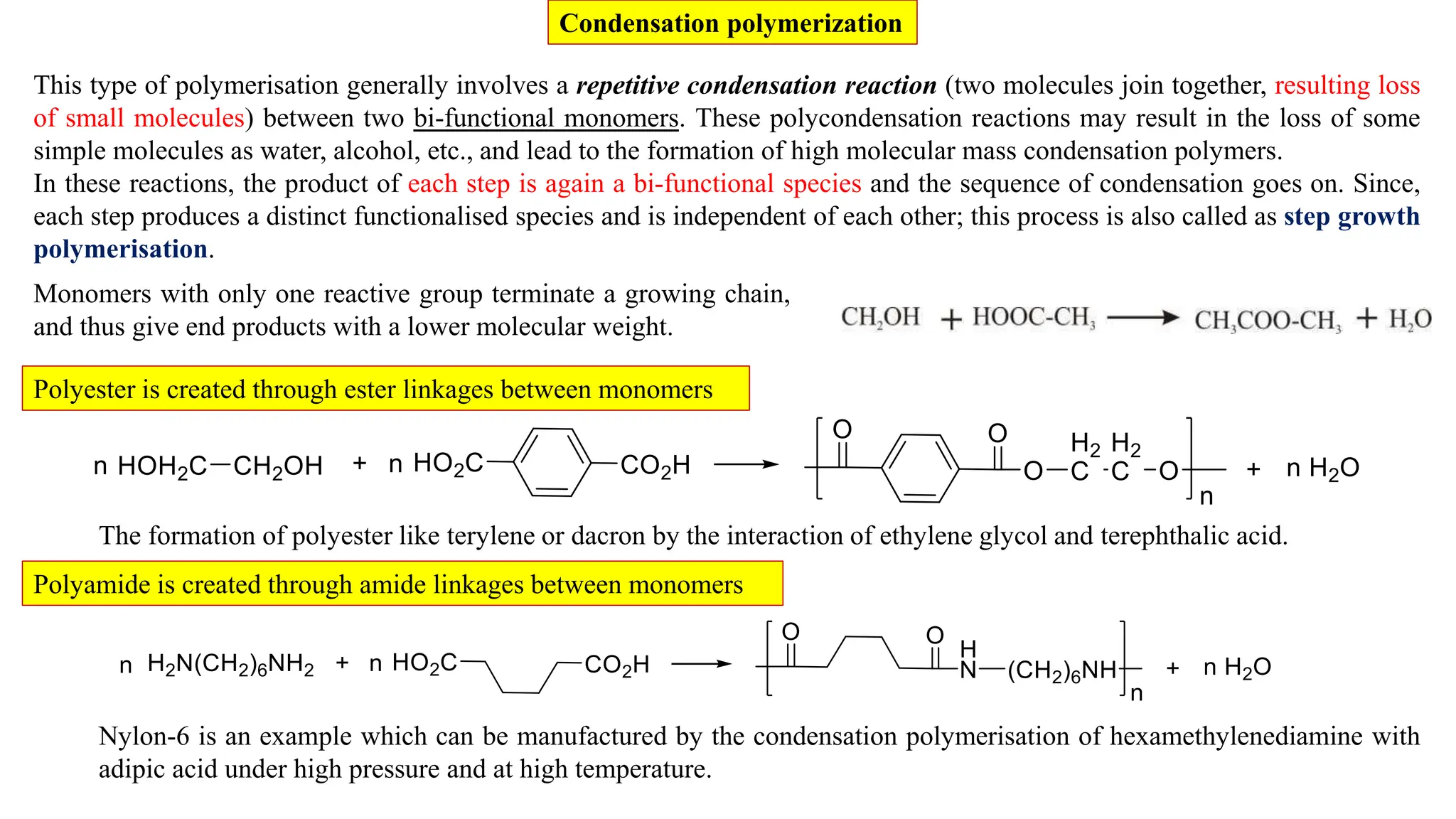
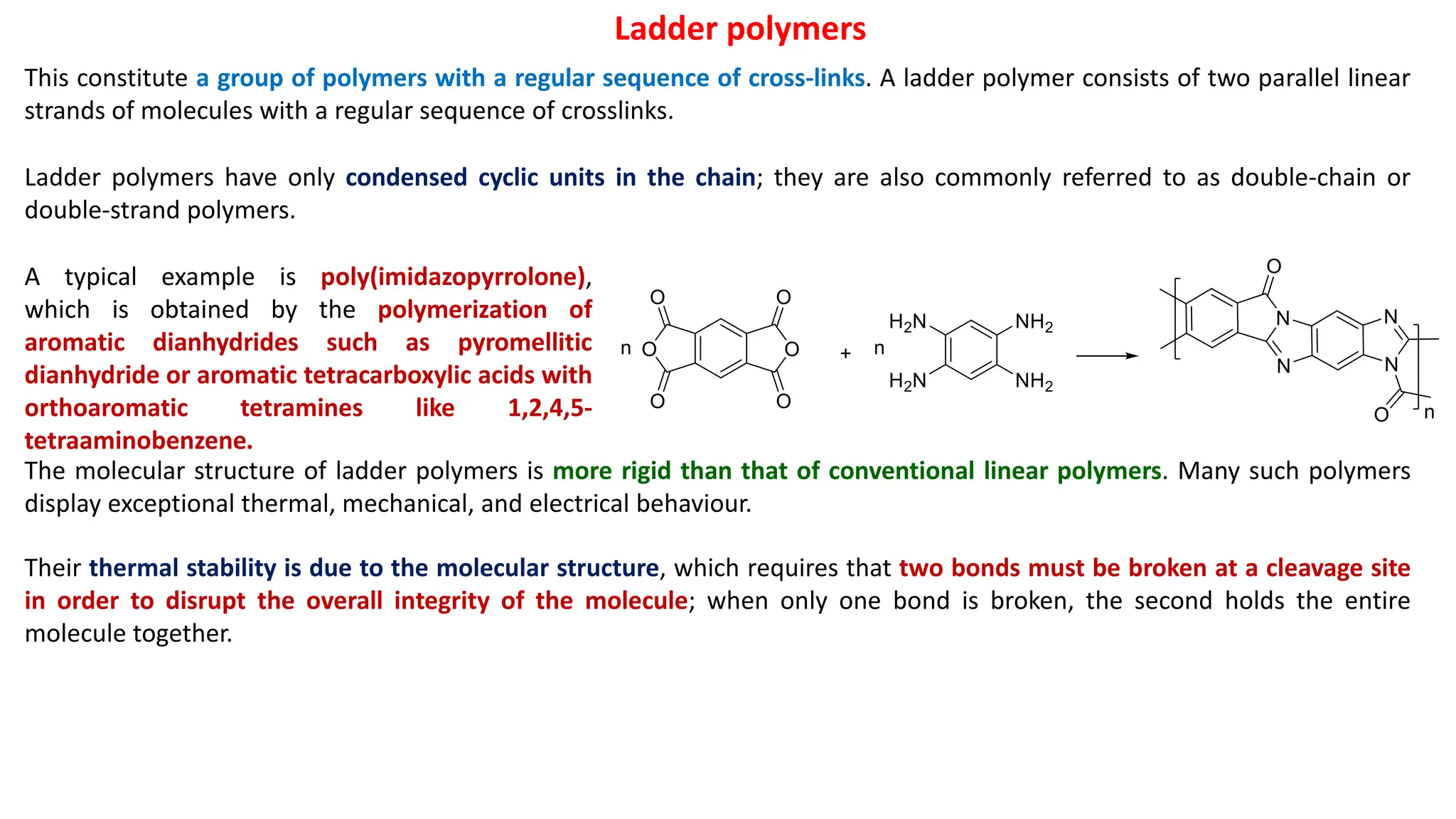
The document discusses various types of polymerization reactions including condensation polymerization, which involves the step-wise reaction of bifunctional monomers to form polymers through the elimination of small molecules like water or alcohol. It also describes different mechanisms of addition polymerization, specifically free radical, cationic, and anionic polymerization which involve the chain growth of polymers through initiation, propagation, and termination steps. Key initiators and mechanisms are outlined for different polymerization reactions.































![Anionic polymerization
Use of anionic initiators which include reagents capable of providing negative ions.
Typical catalysts include sodium in liquid ammonia, alkali metal alkyls, Grignard reagents and triphenylmethyl sodium
[(C6H5)3C-Na].
They are effective with monomers containing electron withdrawing groups like nitrile (–CN) or chloride (-Cl), etc. They
include acrylonitrile [CH2=C(CN)], vinyl chloride [CH2=C(Cl)], methyl methacrylate [CH2=C(CH3)COOCH3], etc.
Ability of substituents to stabilize carbanions decreases as: -NO2 > -C=O > -SO2 > -CO2 ~ -CN > -SO > -Ph ~ -CH=CH2 >>> -CH3.
i) Chain Initiation
Base Initiators are often organometallic compounds or salt of a strong base, such as an alkali metal alkoxide or alacoholates
(tert-BuOLi, tert-BuOK), Sodium metal in tetrahydrofuran, sodium or Potassium with liquid ammonia, Stable alkali metal
complexes may be formed with aromatic compounds (e.g. Na/naphthalene) in ether, alkyl amides such as NaNH2 or KNH2, .
Organolithium salts (n-BuLi, sec-BuLi, tert-BuLi), Grignard reagent
Potassium amide (K+NH2
-) adds to C – C double bond of alkene to form stable carbanion. Where, W is electron withdrawing
group.](https://image.slidesharecdn.com/polymerisation-240129030409-aff782fb/75/Polymerisation-reactions-and-synthesis-of-important-polymers-35-2048.jpg)



