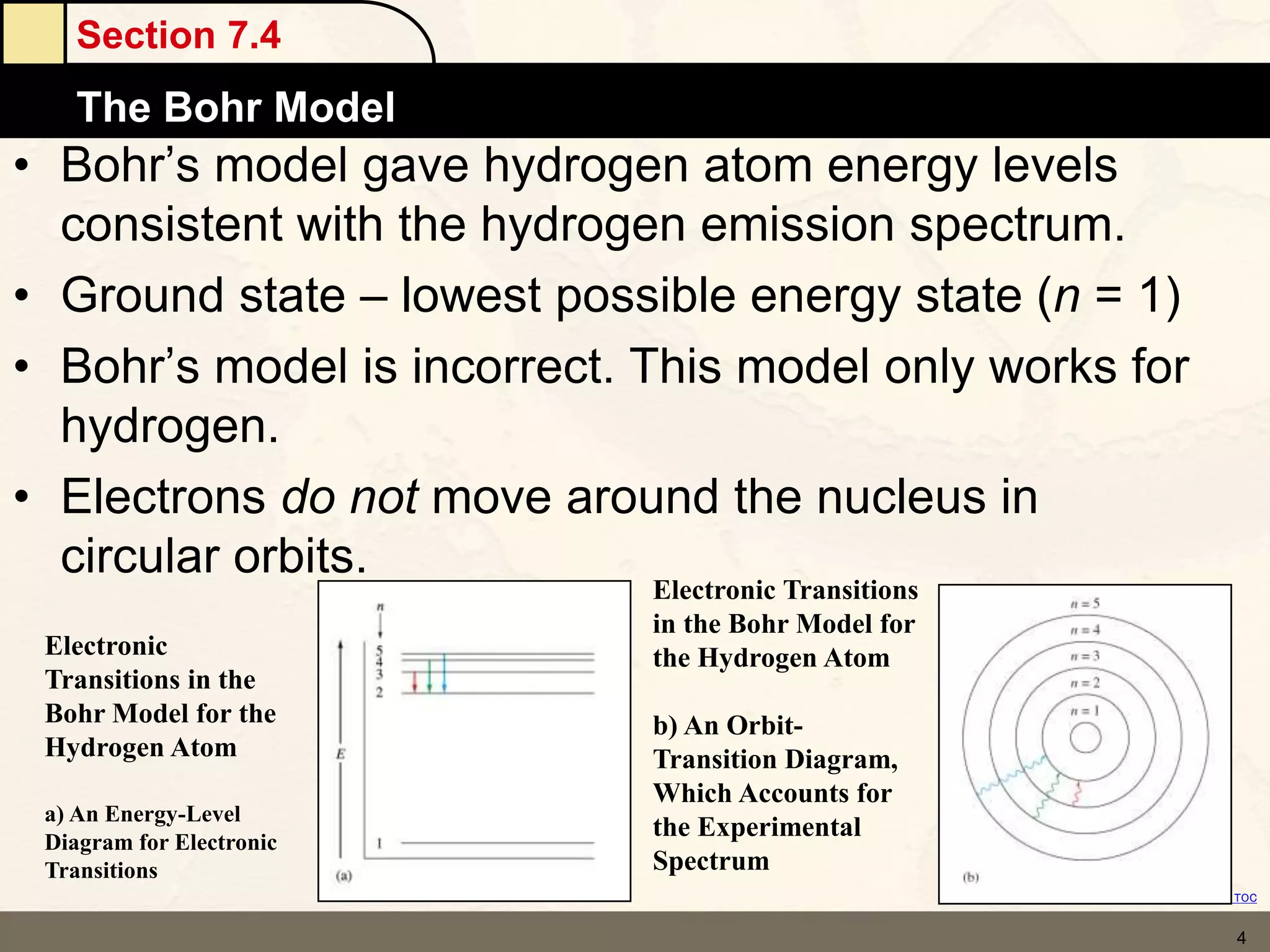
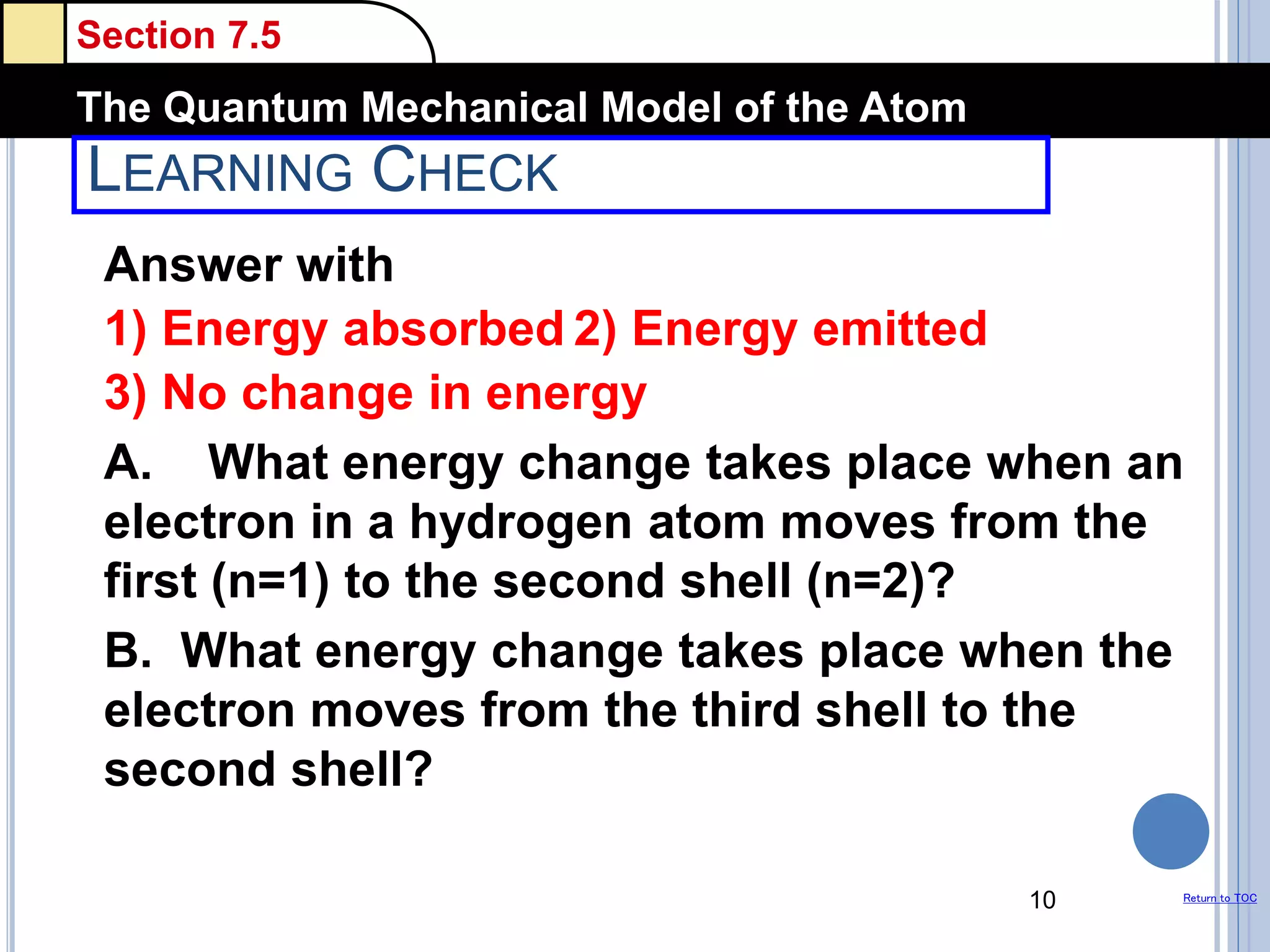
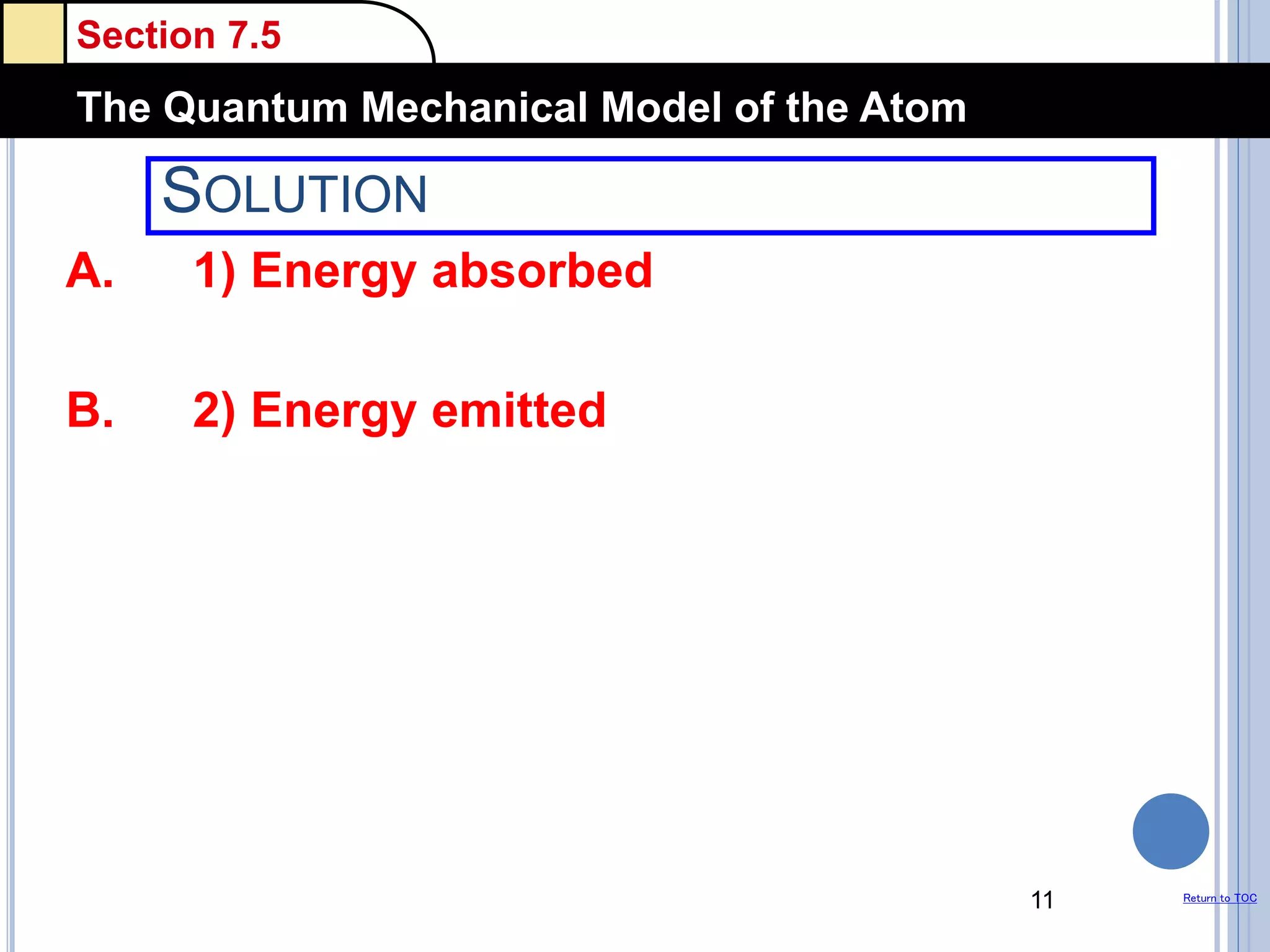
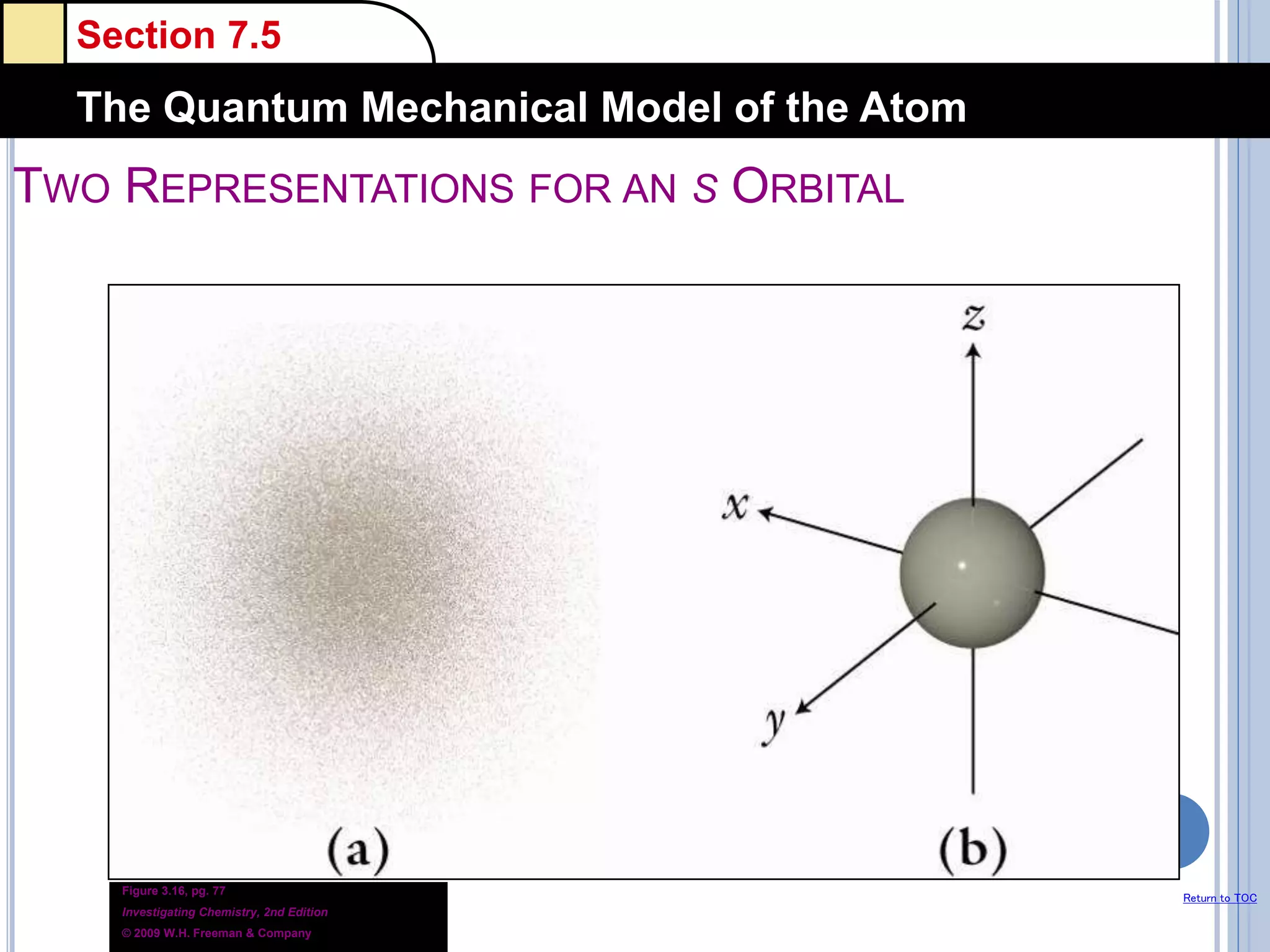
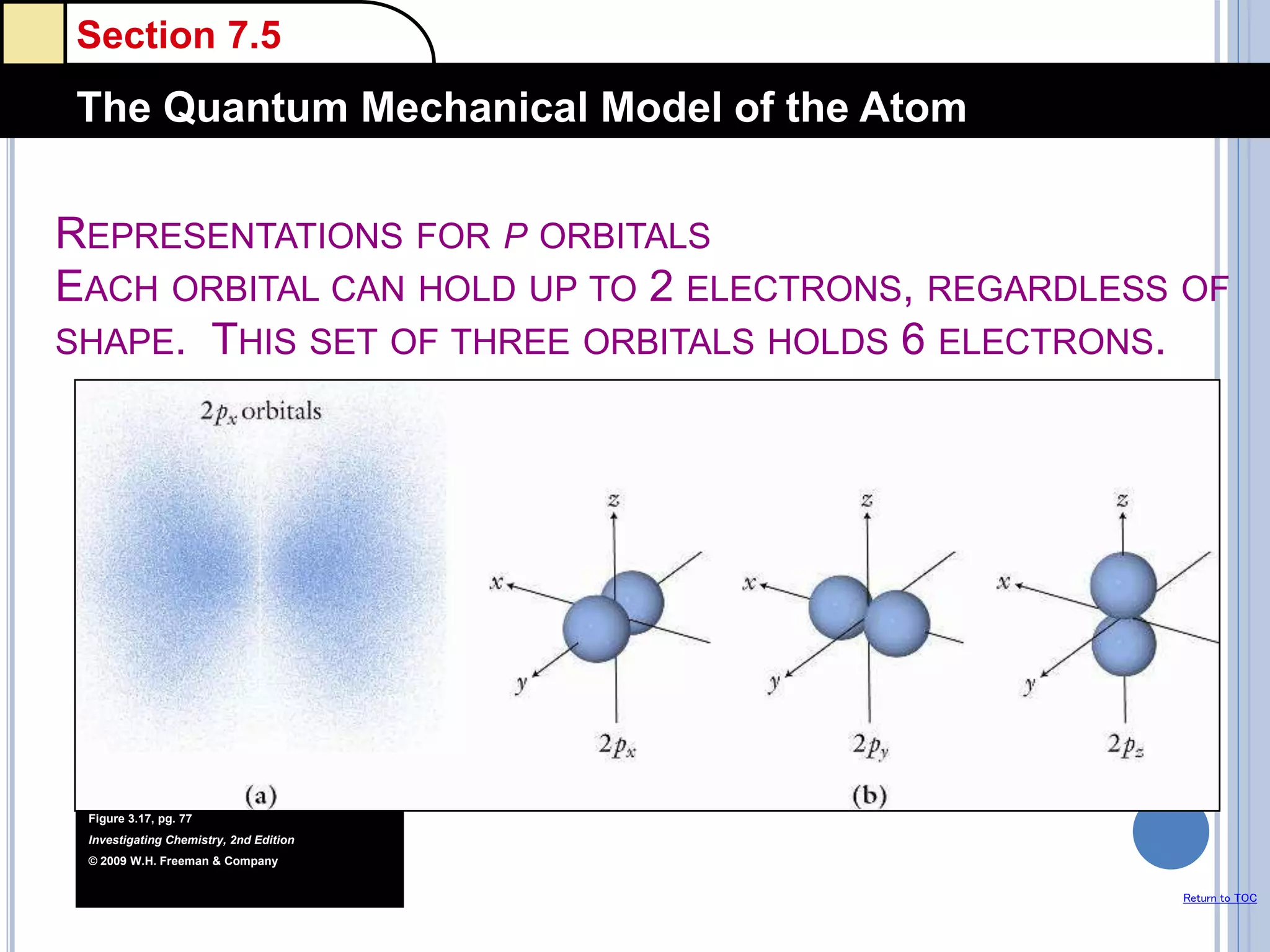
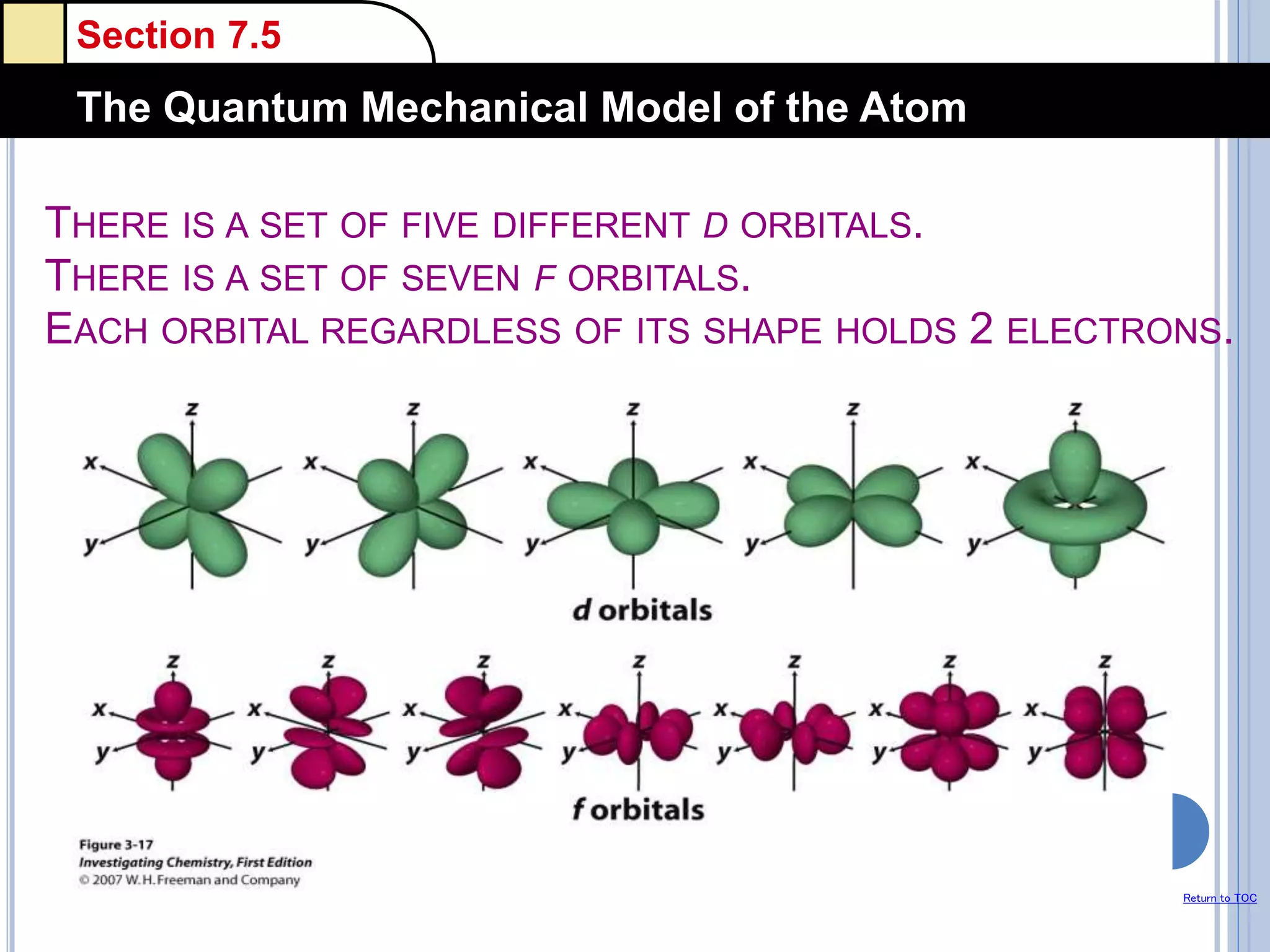
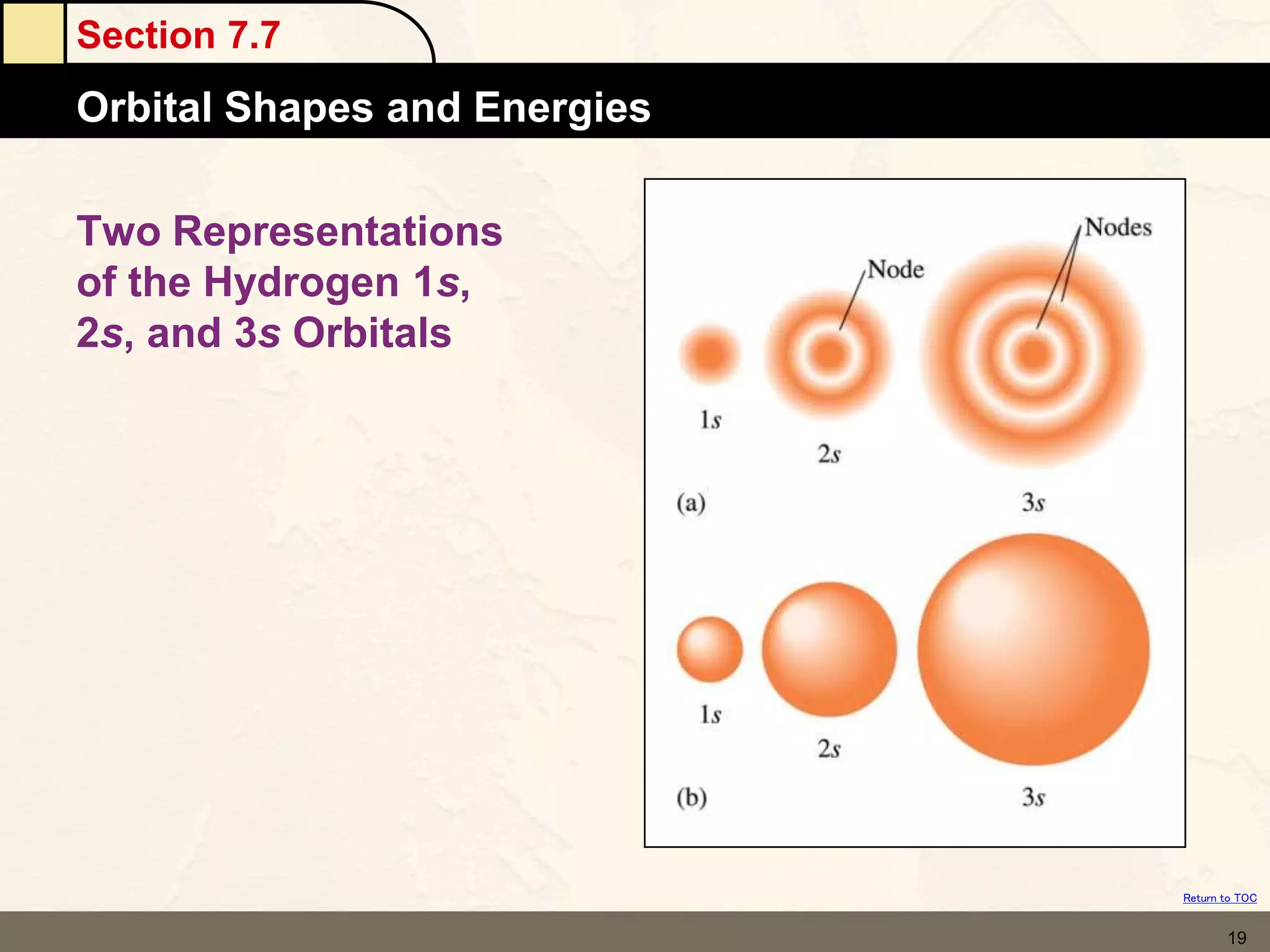
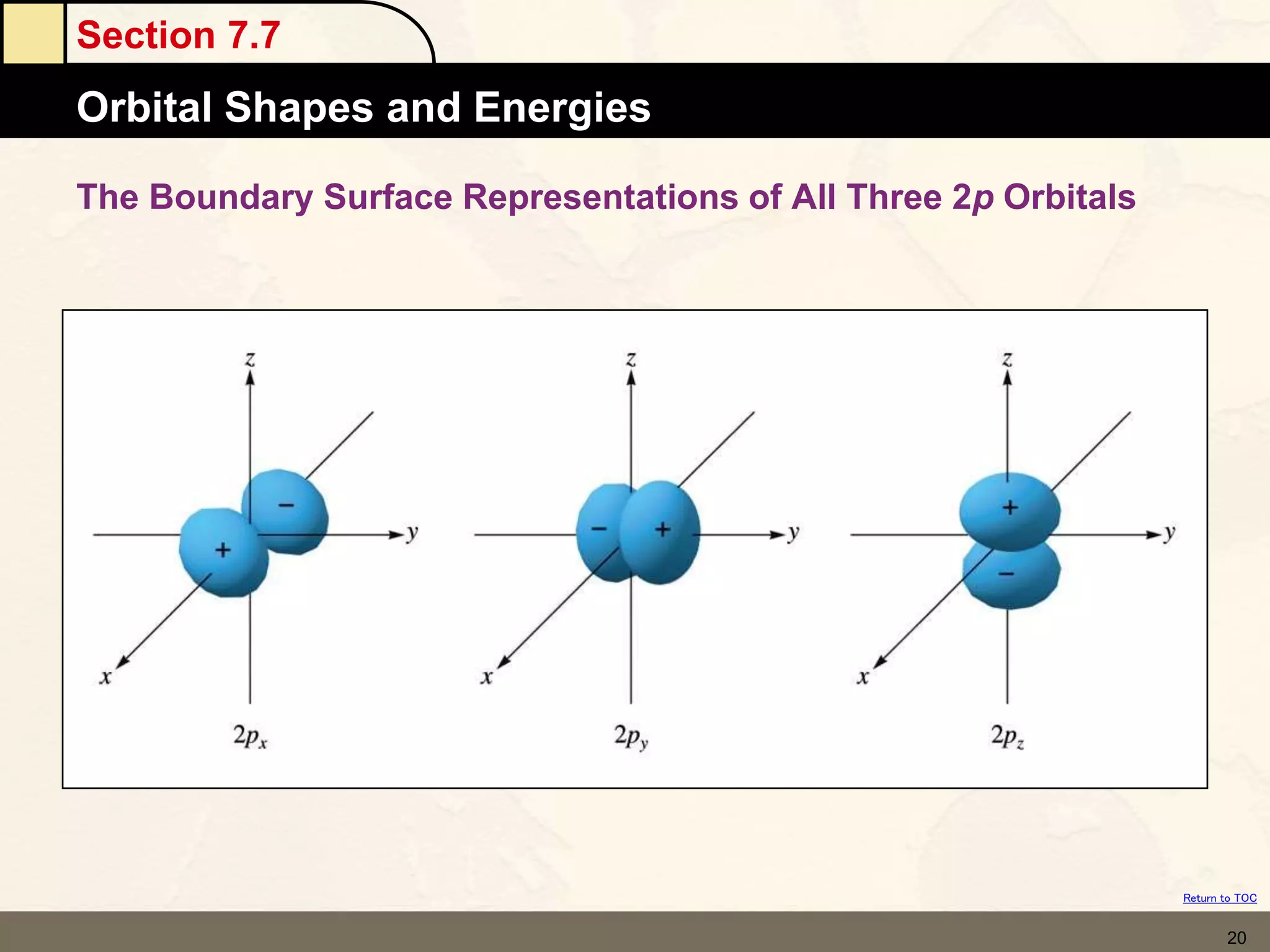
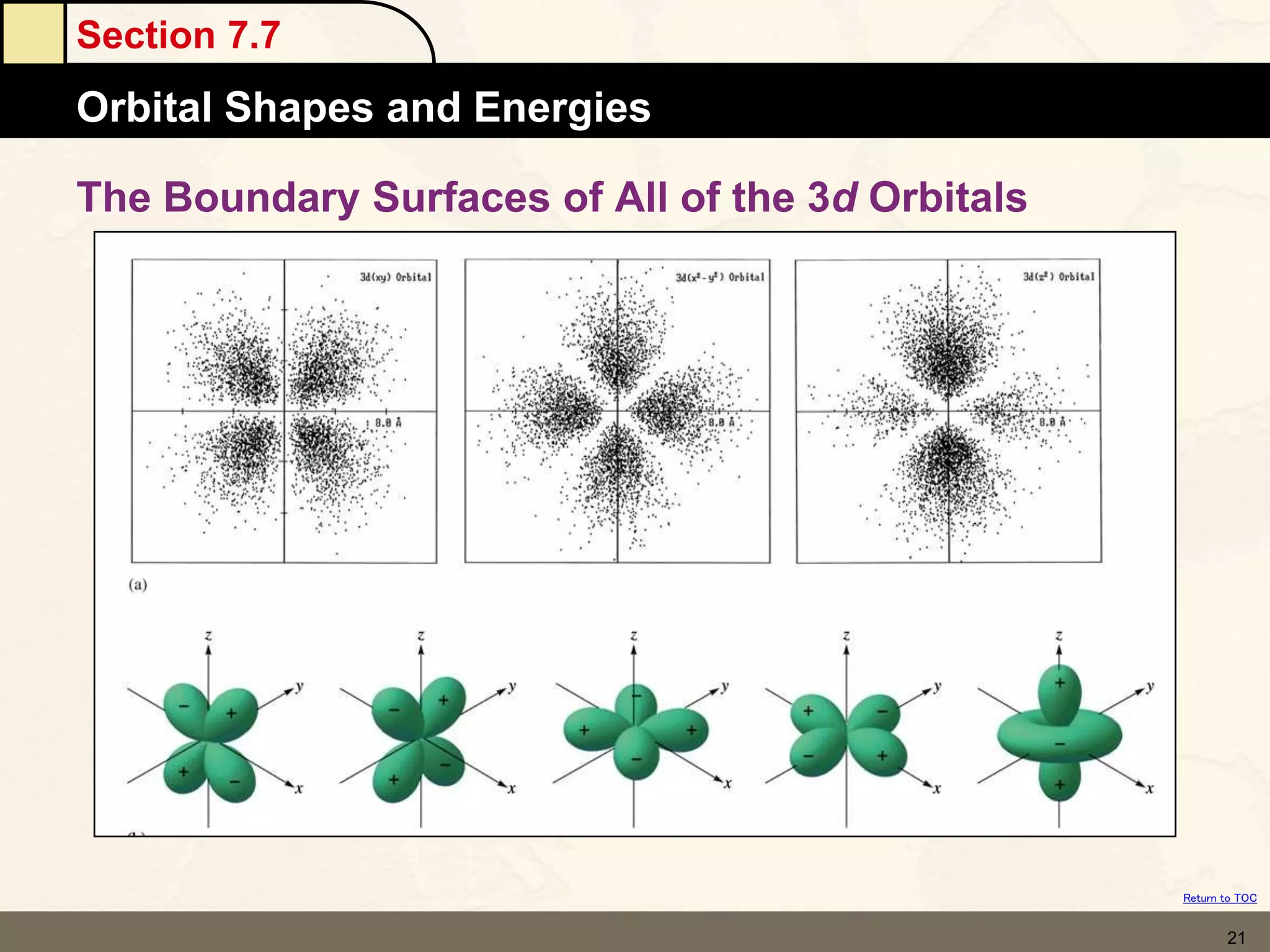
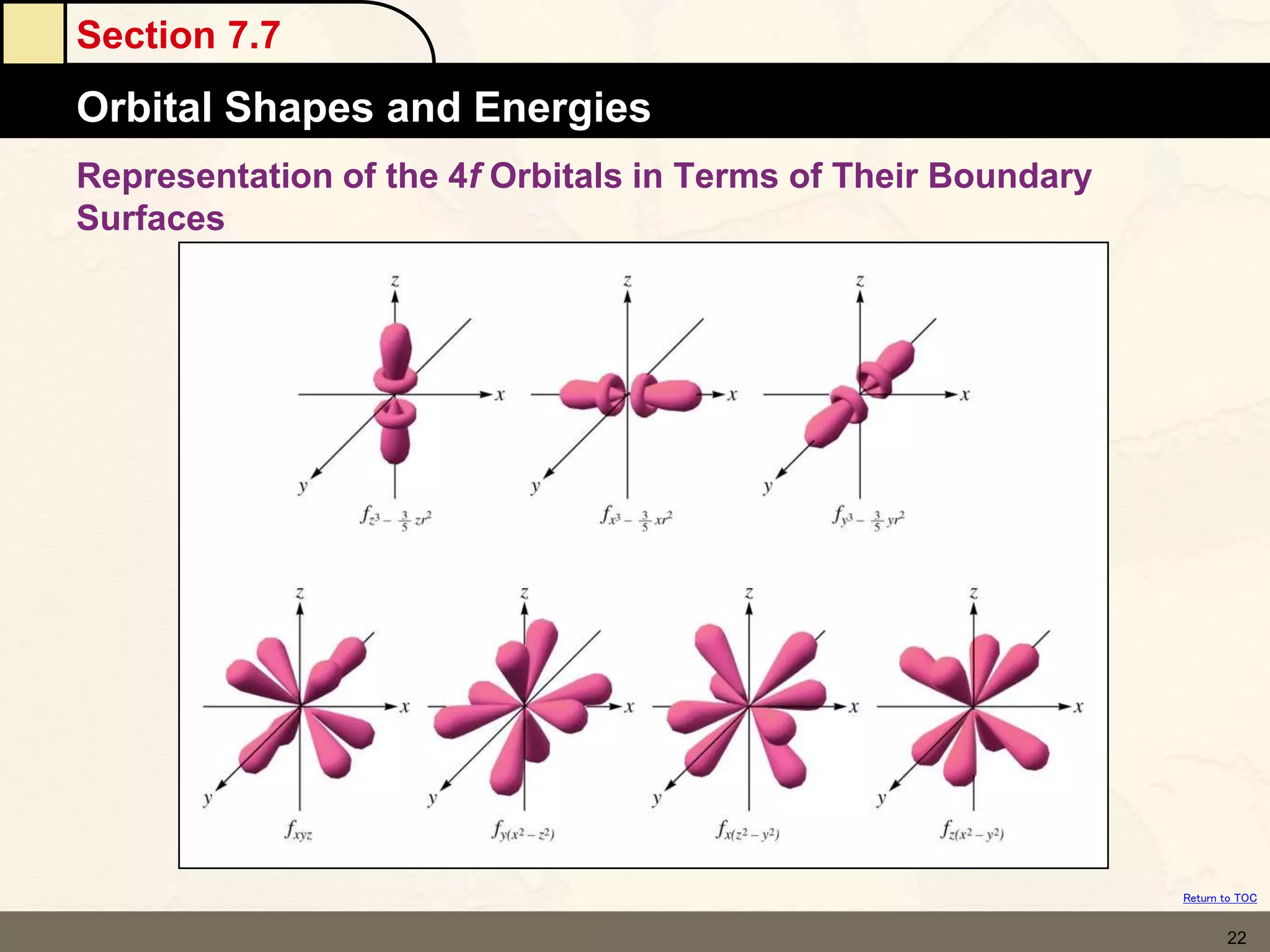
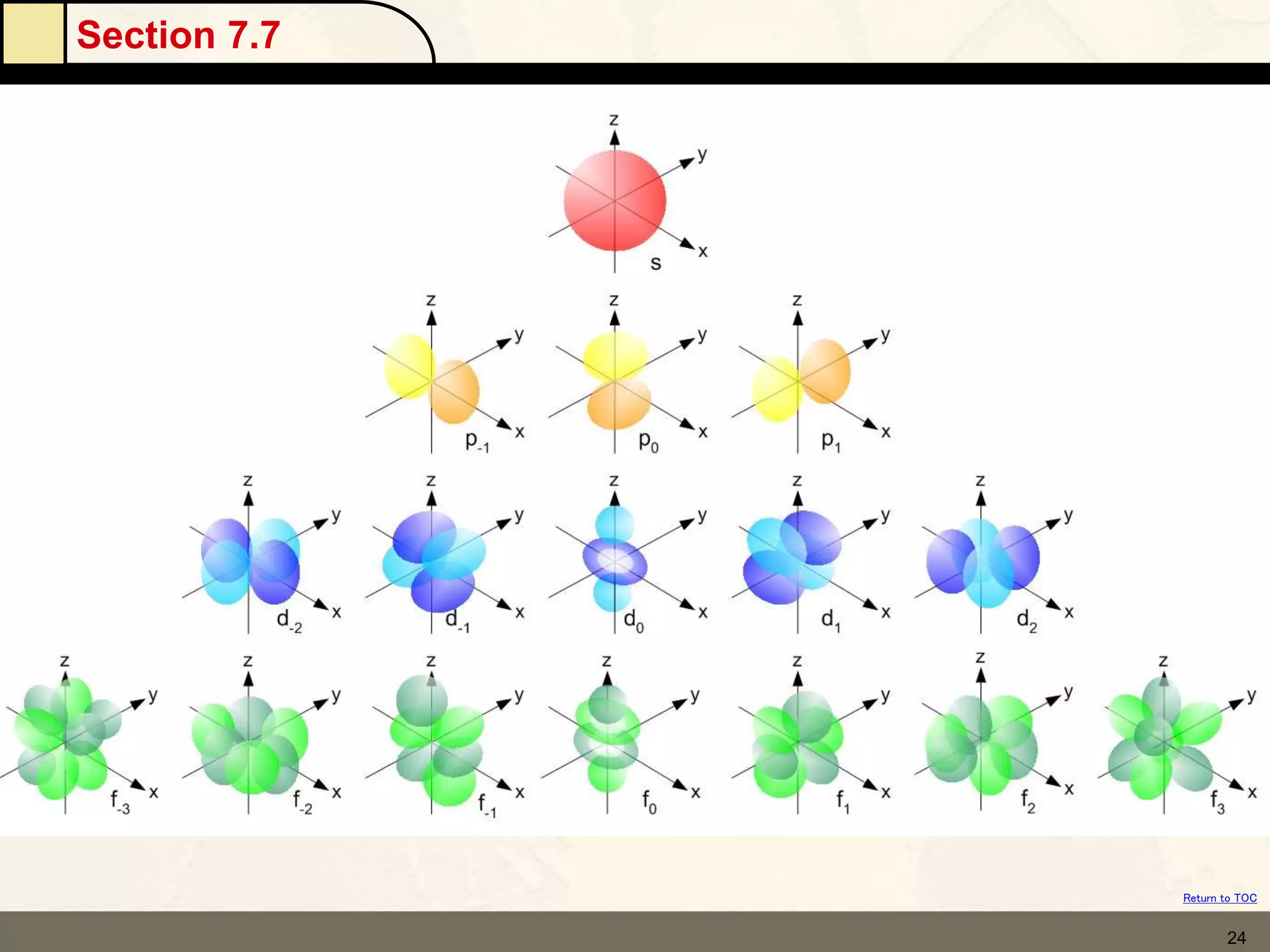
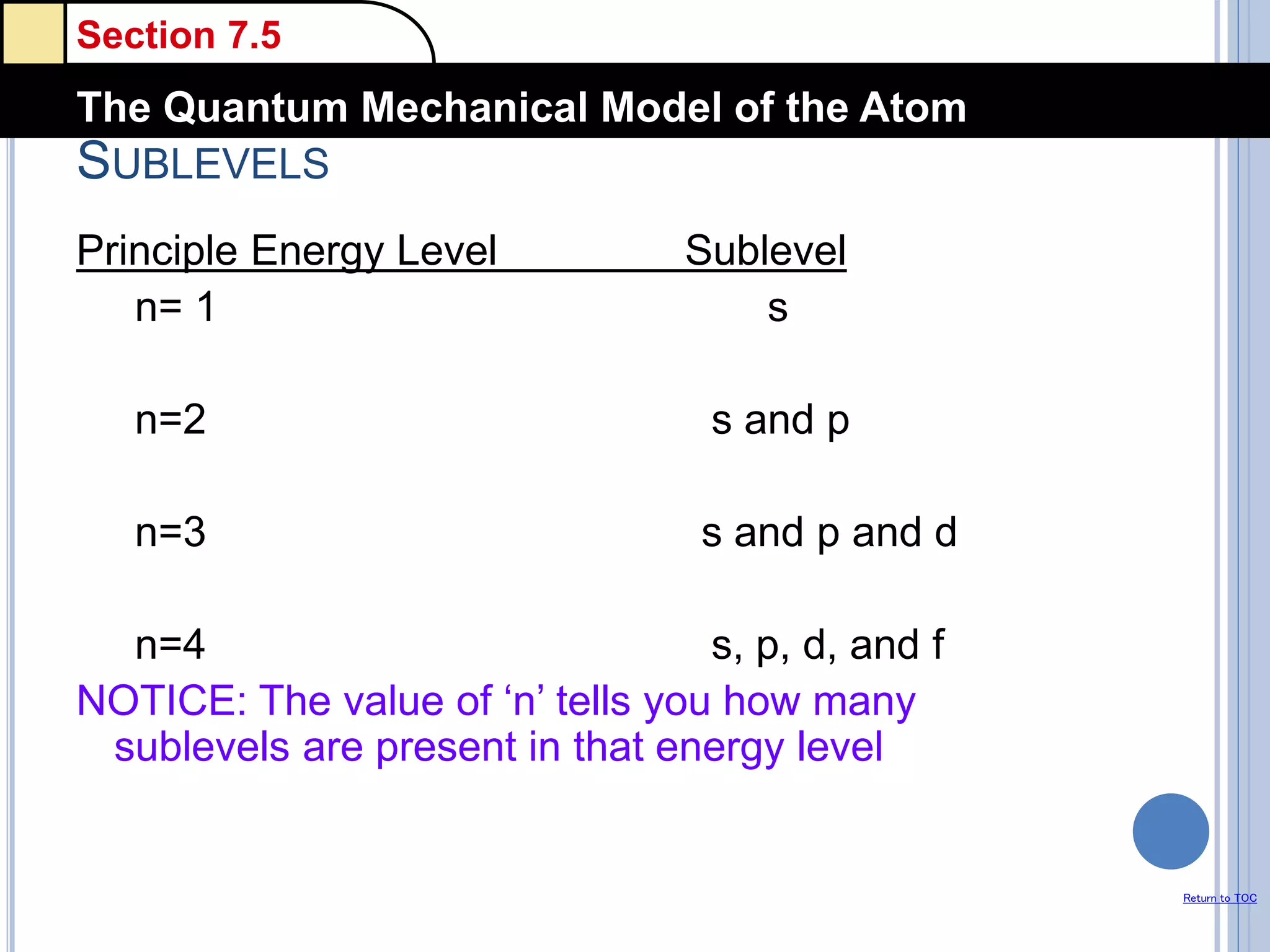
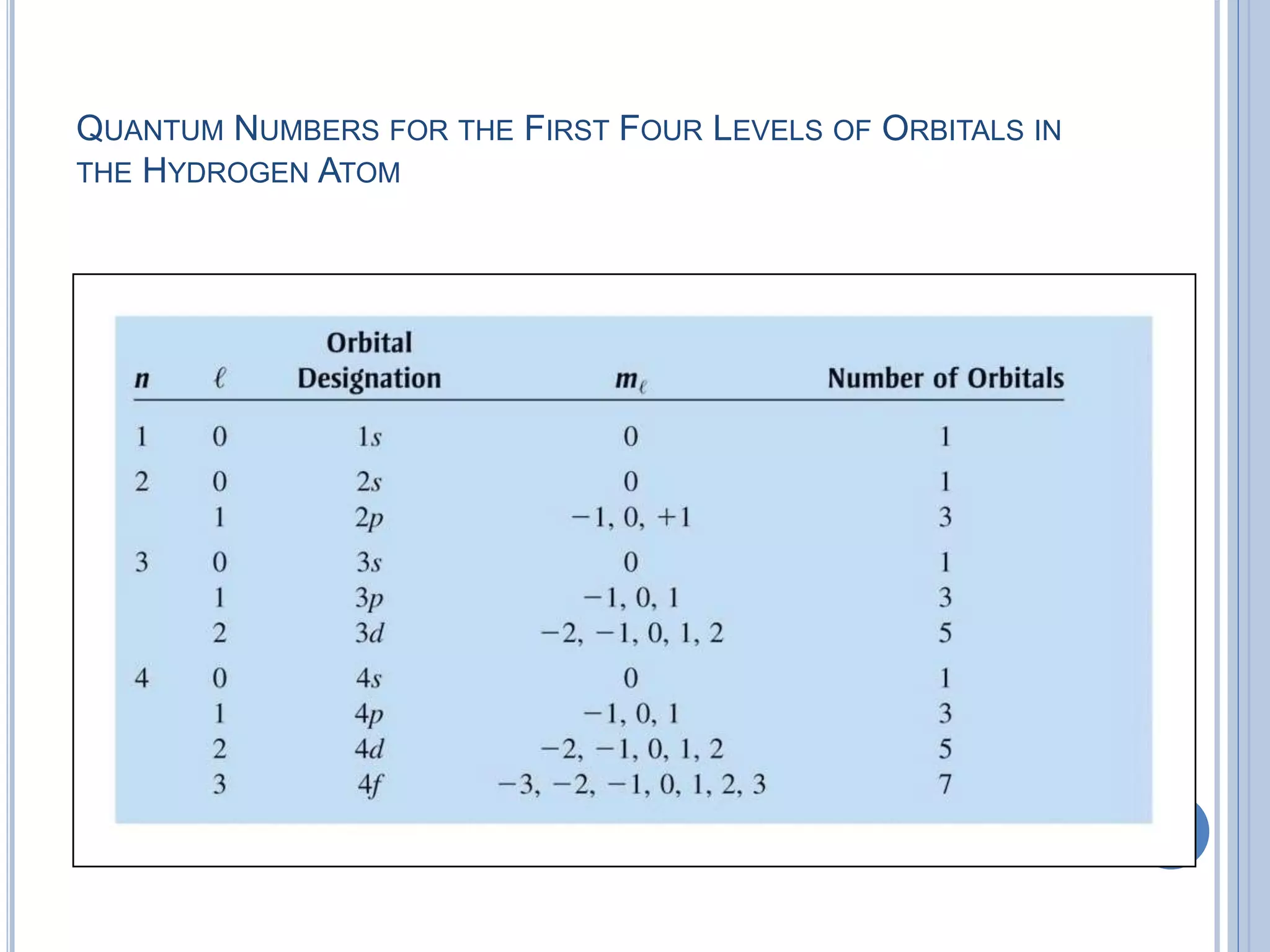
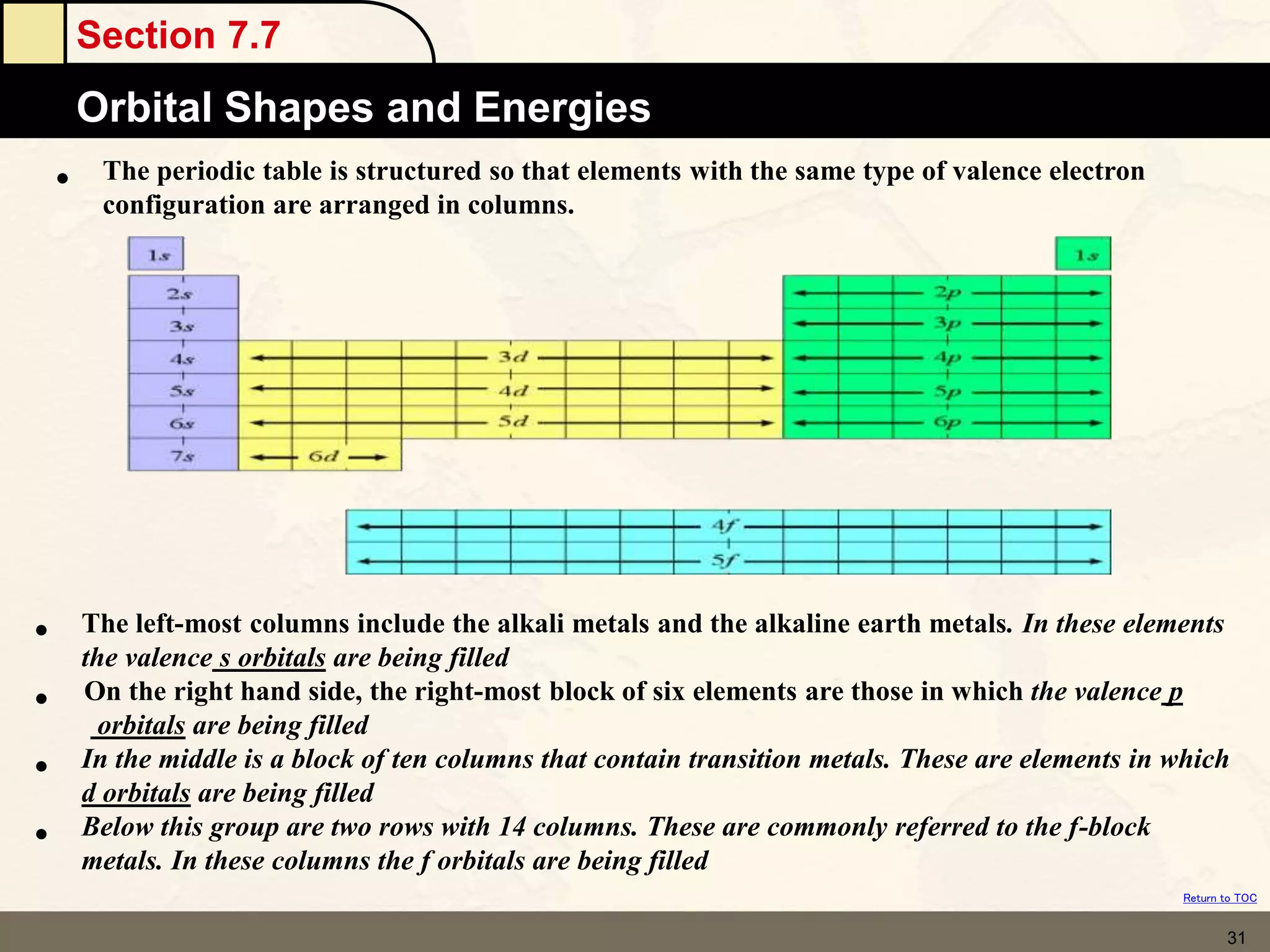
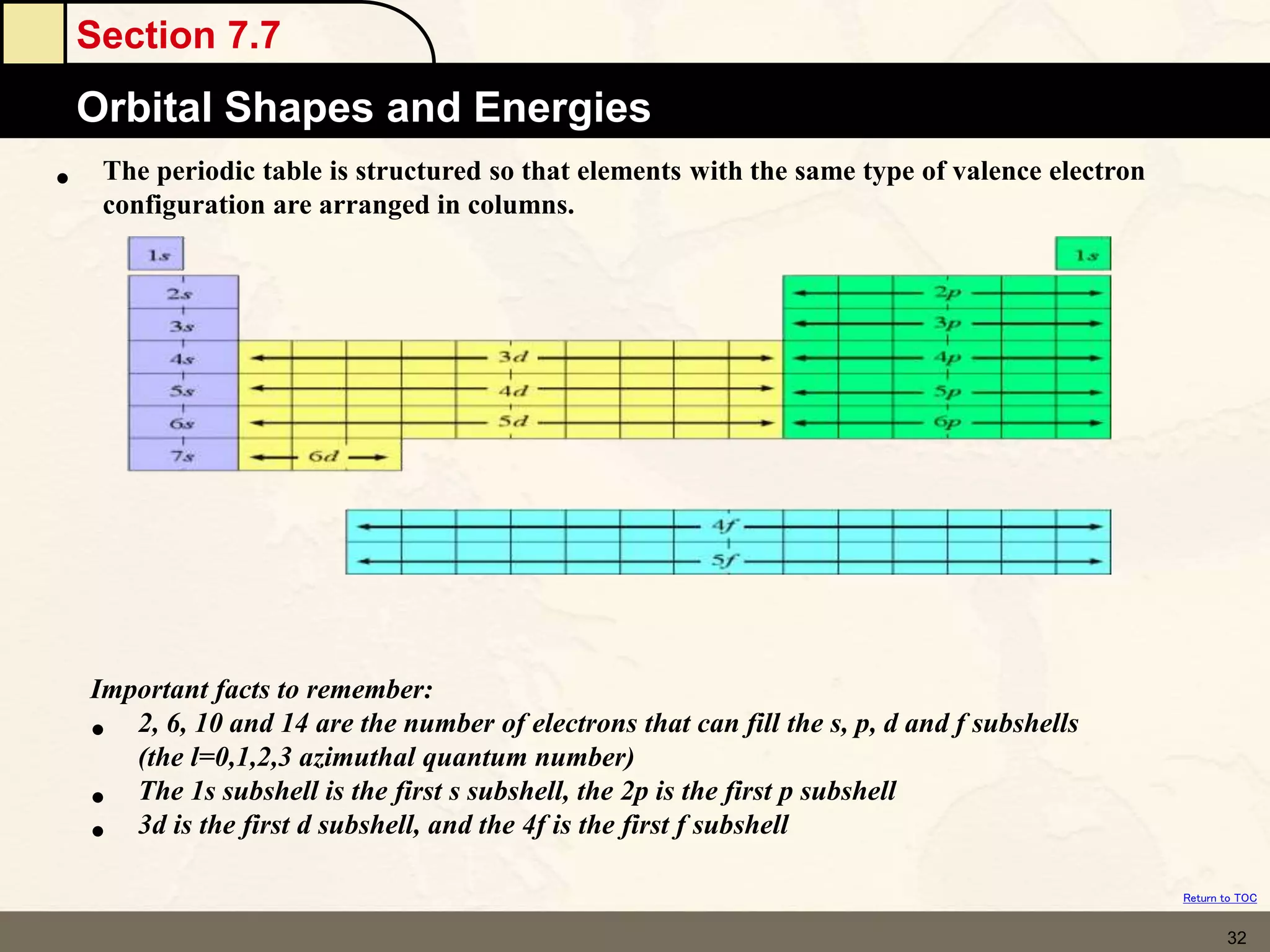
The document discusses different models of the atom, including Bohr's model and the quantum mechanical model. Bohr's model proposed that electrons orbit the nucleus in fixed orbits, but this was later found to be incorrect. According to the quantum mechanical model, electrons move in regions of probability called orbitals around the nucleus. Orbitals are defined using quantum numbers, which describe the electron's energy level, orbital shape, and orientation in space. Electrons are arranged into discrete energy levels and can move between levels by absorbing or emitting energy.