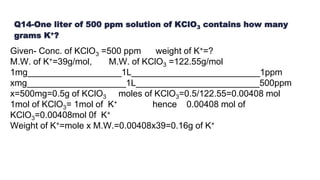
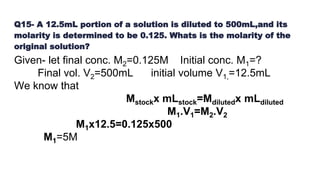
This document summarizes a seminar on concentration of solutions presented by Miss. Neha Milind Dhansekhar. It defines concentration and discusses various units used to express concentration, including percentage by mass and volume, molarity, molality, and ppm/ppb. It provides examples of calculating concentration using these units and converting between units. Common dilution calculations and sample problems determining the mass or moles of solute required for a given concentration are presented.

![Concentration of solution
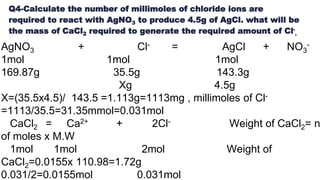
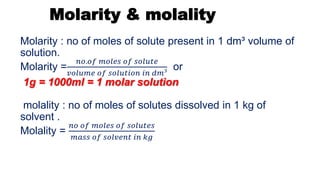
Definition : The concentration is defined as amount of solute
dissolved in a specific amount of solvent.
Concentration of solution expressed in different ways :
1. Percentage by mass : [ w/w ]
2. Percentage by volume : [ v/v ]
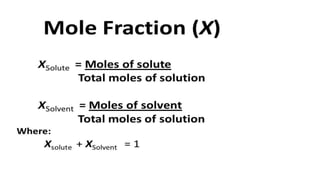
3. Molefraction
4. Molarity
5. Molality
6. Normality
7. Formality , etc.](https://image.slidesharecdn.com/ppmppbppt1-190428140820/85/chemometrics-2-320.jpg)
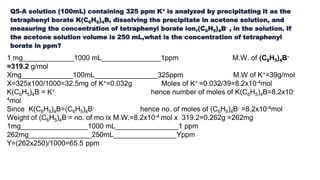
![Percentage by mass & volume
Percentage by mass [w/w] =
𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒
𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛
×100
Percentage by volume [ v/v ]=
𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒
𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛
× 100
Percentage by weight by volume
[w/v ]=
𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒
(𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛)
× 100](https://image.slidesharecdn.com/ppmppbppt1-190428140820/85/chemometrics-3-320.jpg)


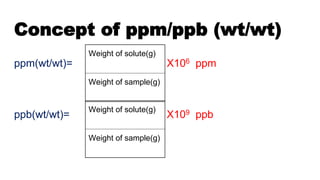
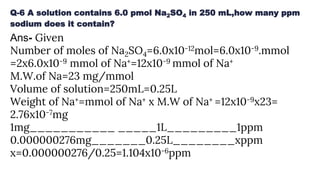
![Concept of ppm/ppb (wt/vol)
ppm is an abbreviation for "parts per million" and
it also can be expressed
as milligrams per liter (mg/L).
Parts per billion (ppb) is the number of units of
mass of a contaminant per 1000 million units of
total mass. Also µg/L or micrograms per liter
ppm(wt/vol)= X106 ppmWeight of solute(g)
Volume of solution [ L ]
Weight of solute(g)
Volume of sample(mL)
ppb(wt/vol)= ×10⁹](https://image.slidesharecdn.com/ppmppbppt1-190428140820/85/chemometrics-9-320.jpg)