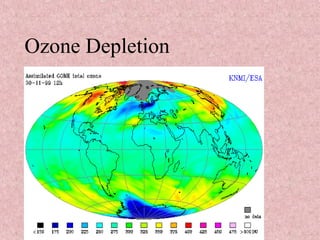
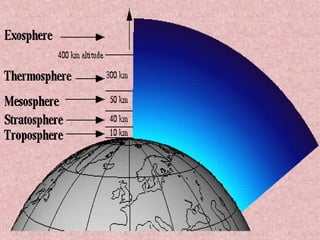
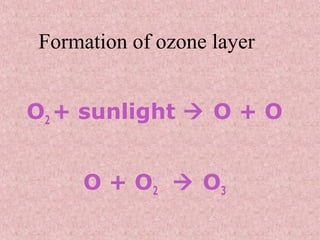
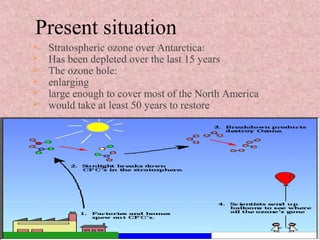
The document discusses ozone depletion and the importance of the ozone layer. It explains that the ozone layer is found in the stratosphere between 10-50km above the Earth's surface and protects the surface from harmful UV radiation. It also discusses how CFCs released into the atmosphere were depleting the ozone layer by causing a chemical reaction that breaks down ozone molecules. As a result of the thinning ozone layer, more UV radiation reaches the Earth's surface, increasing risks of skin cancer, eye disease, and damage to food crops and marine life.