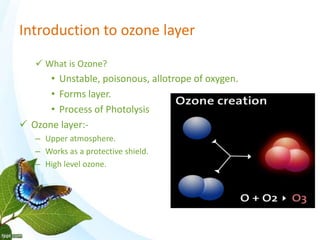
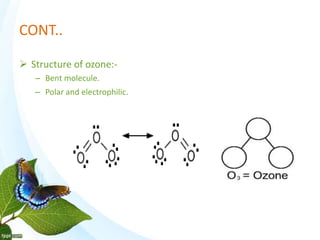
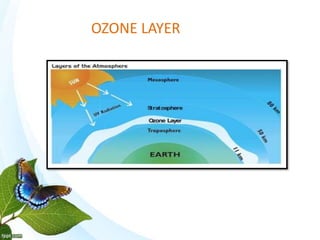
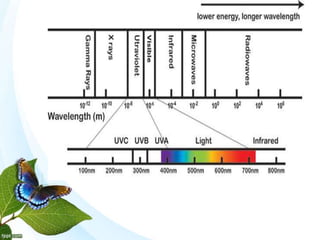
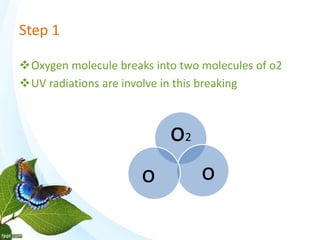
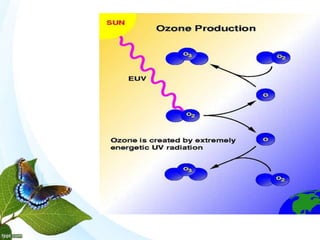
The document discusses the ozone layer, its formation and location in the stratosphere. It protects life on Earth by absorbing UV radiation. Ozone is formed through chemical reactions involving oxygen and UV radiation. Depletion of the ozone layer is caused by catalytic cycles involving halogen gases like chlorine and bromine, which destroy ozone molecules. This increased UV radiation reaching the Earth's surface and can harm humans, animals, and ecosystems. Global warming is caused by increased greenhouse gases trapping heat, but is a separate issue from ozone depletion.