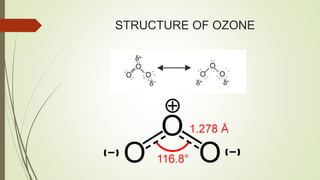
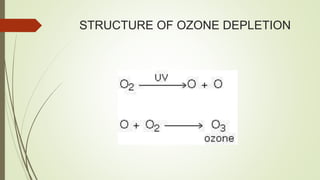
This document summarizes a seminar on ozone depletion presented by Ravi Singh Jadaun on March 23, 2017 at Jiwanji University in Gwalior, India. It defines the ozone layer, discusses its structure and history. It then explains the causes of ozone depletion, both natural and man-made, focusing on ozone depleting substances like CFCs. The effects of depletion on human health, the environment, marine life and animals are outlined. Finally, solutions to reduce depletion like banning pesticides and using public transportation are proposed.