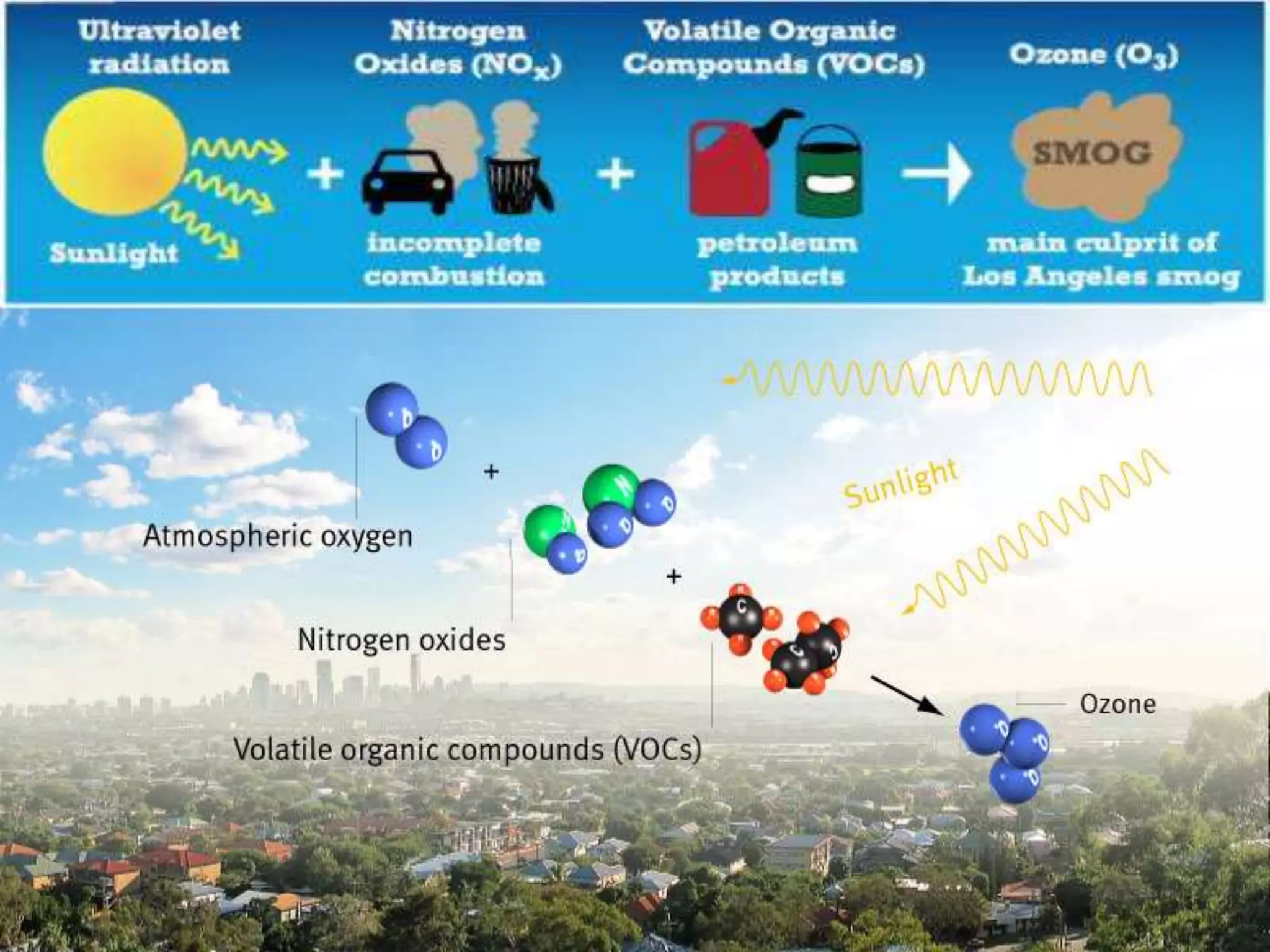
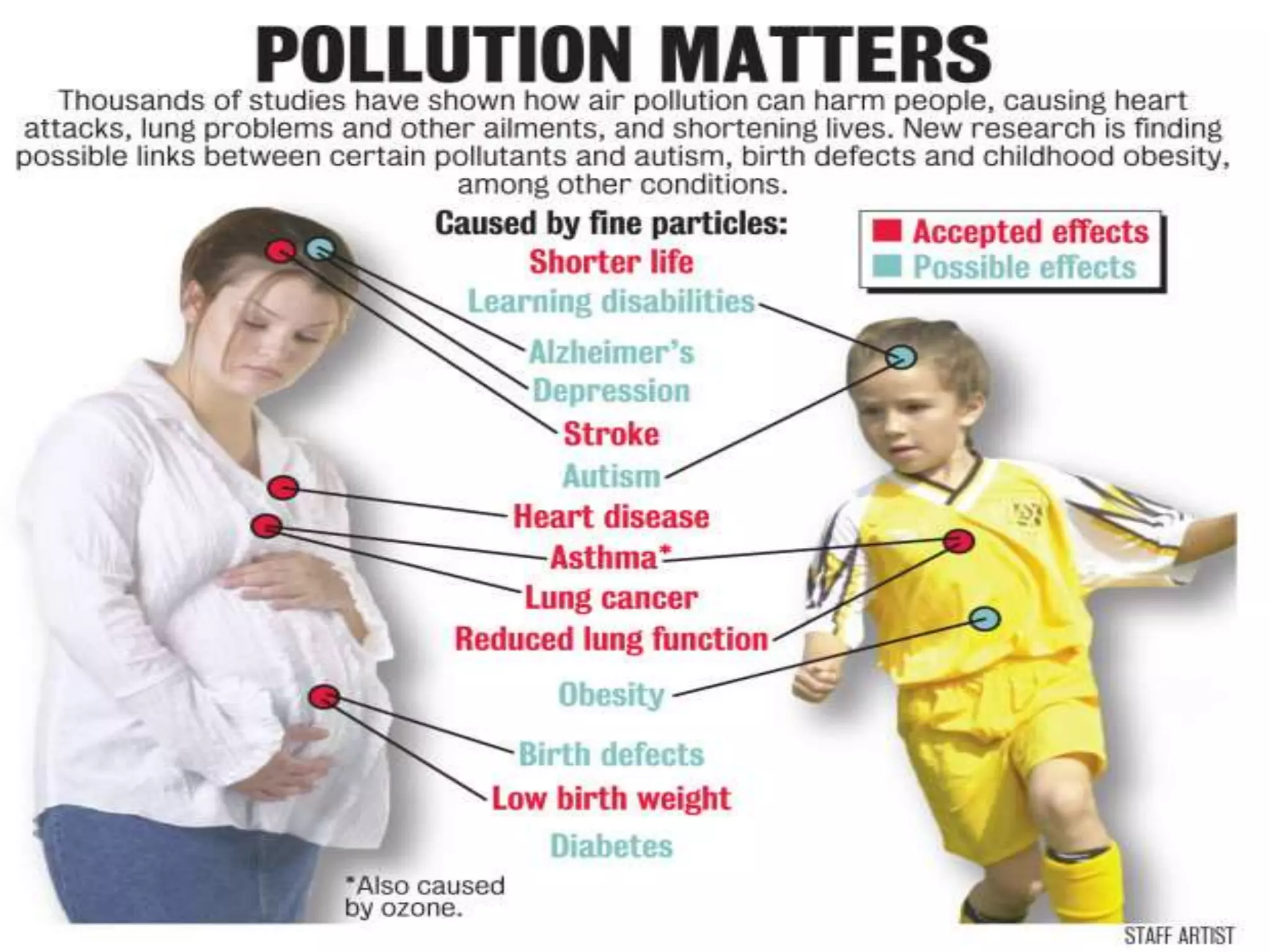
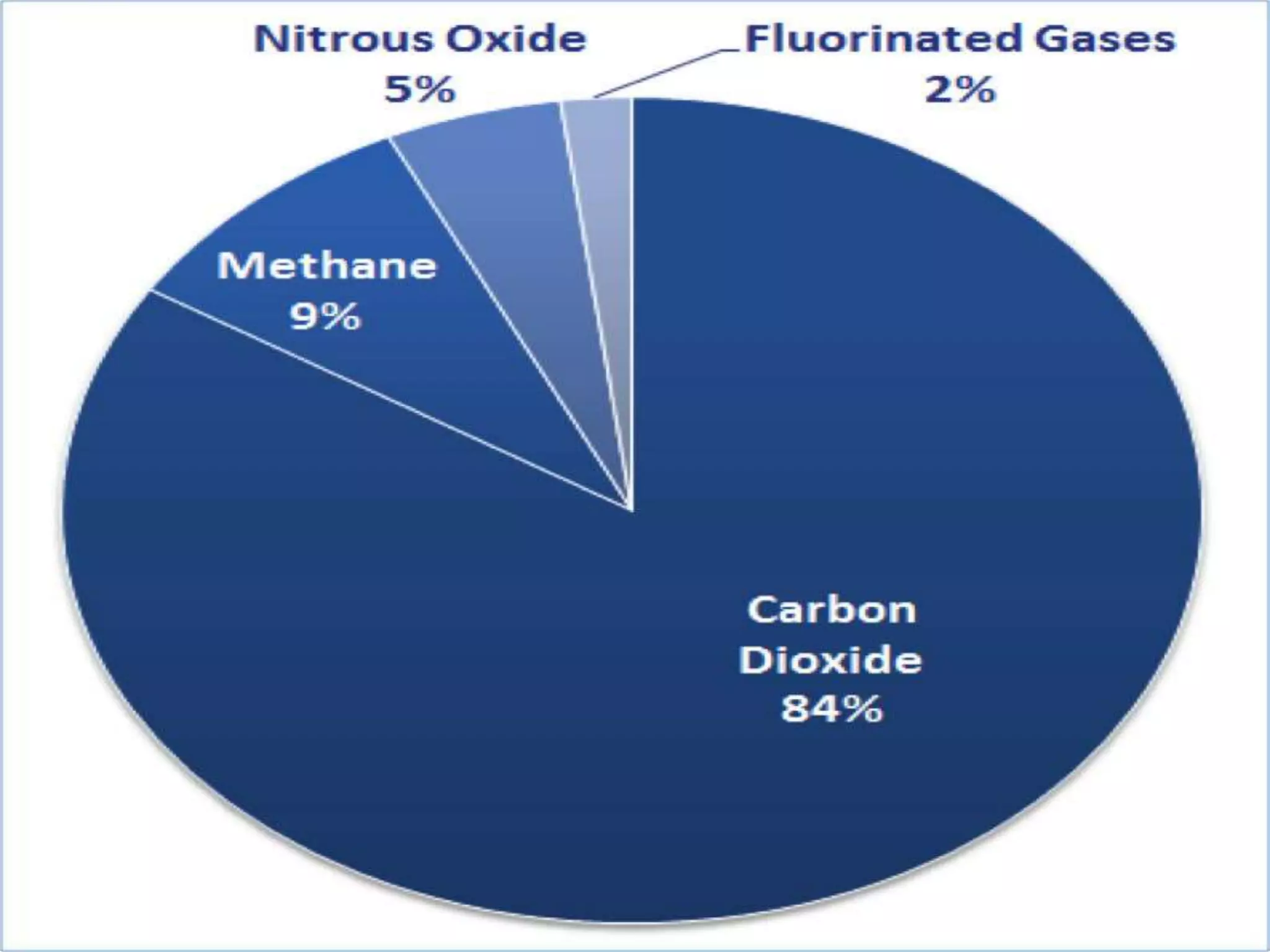
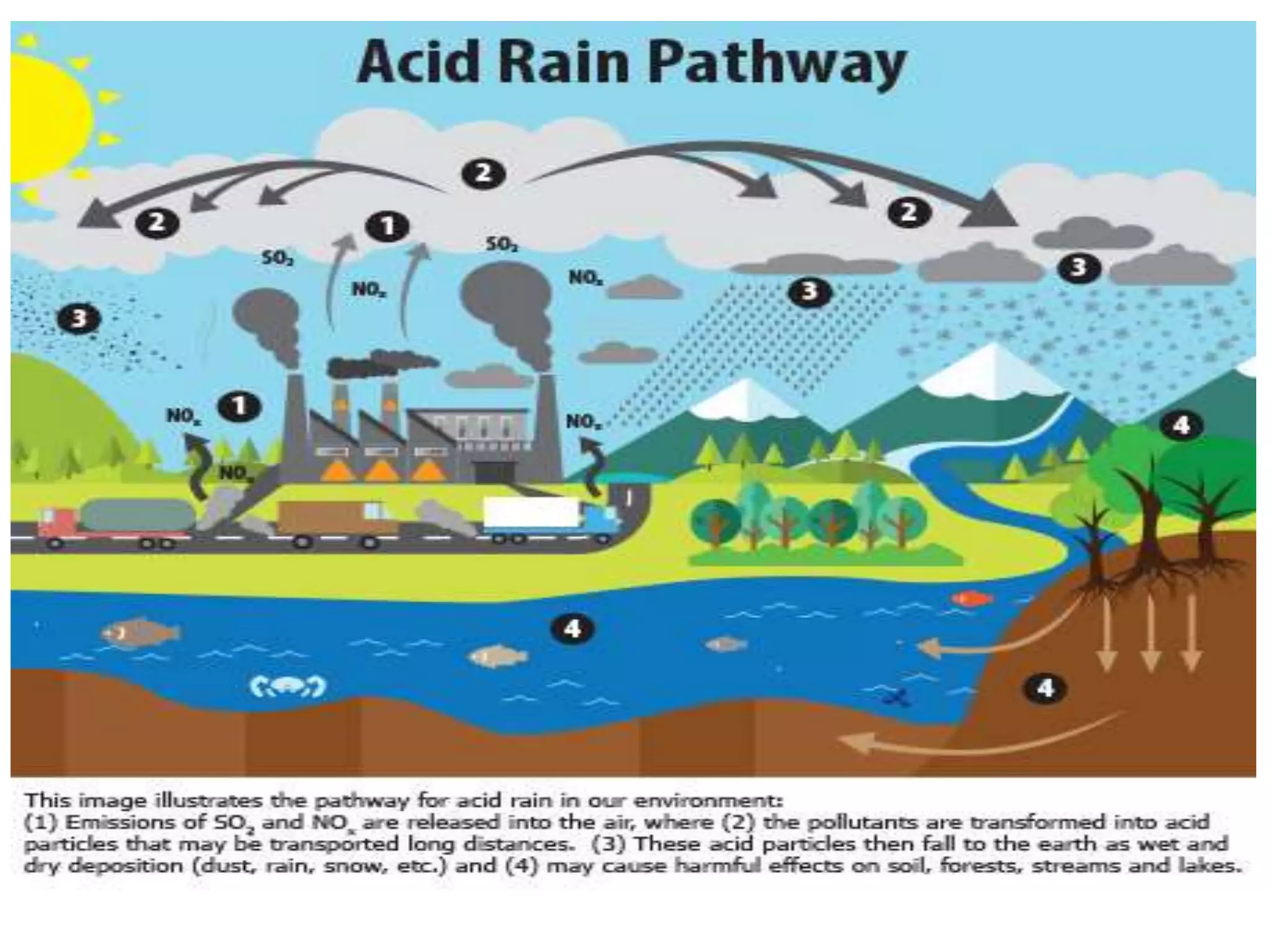
This document discusses various types of automotive emissions and their effects. It describes the three main types of vehicles used in the country and the different types of emissions from gasoline and diesel engines. It then explains vehicle emission control and methods to reduce exhaust emissions, such as modifications to the engine and exhaust gas treatment. Finally, it discusses several types of air pollution caused by vehicle emissions, including nitrogen oxides, smog, and their health effects.