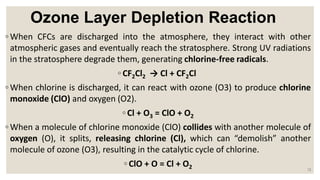
The document provides an overview of the Earth's atmosphere, detailing its four main layers: the troposphere, stratosphere (where the ozone layer exists), mesosphere, and thermosphere, along with the boundary debate regarding the exosphere. It focuses on the formation, function, and depletion of the ozone layer, primarily due to chlorofluorocarbons (CFCs) and their destructive impact on ozone. The Montreal Protocol is highlighted as a successful international treaty aimed at protecting the ozone layer, showing signs of recovery due to reduced ozone-depleting substances.