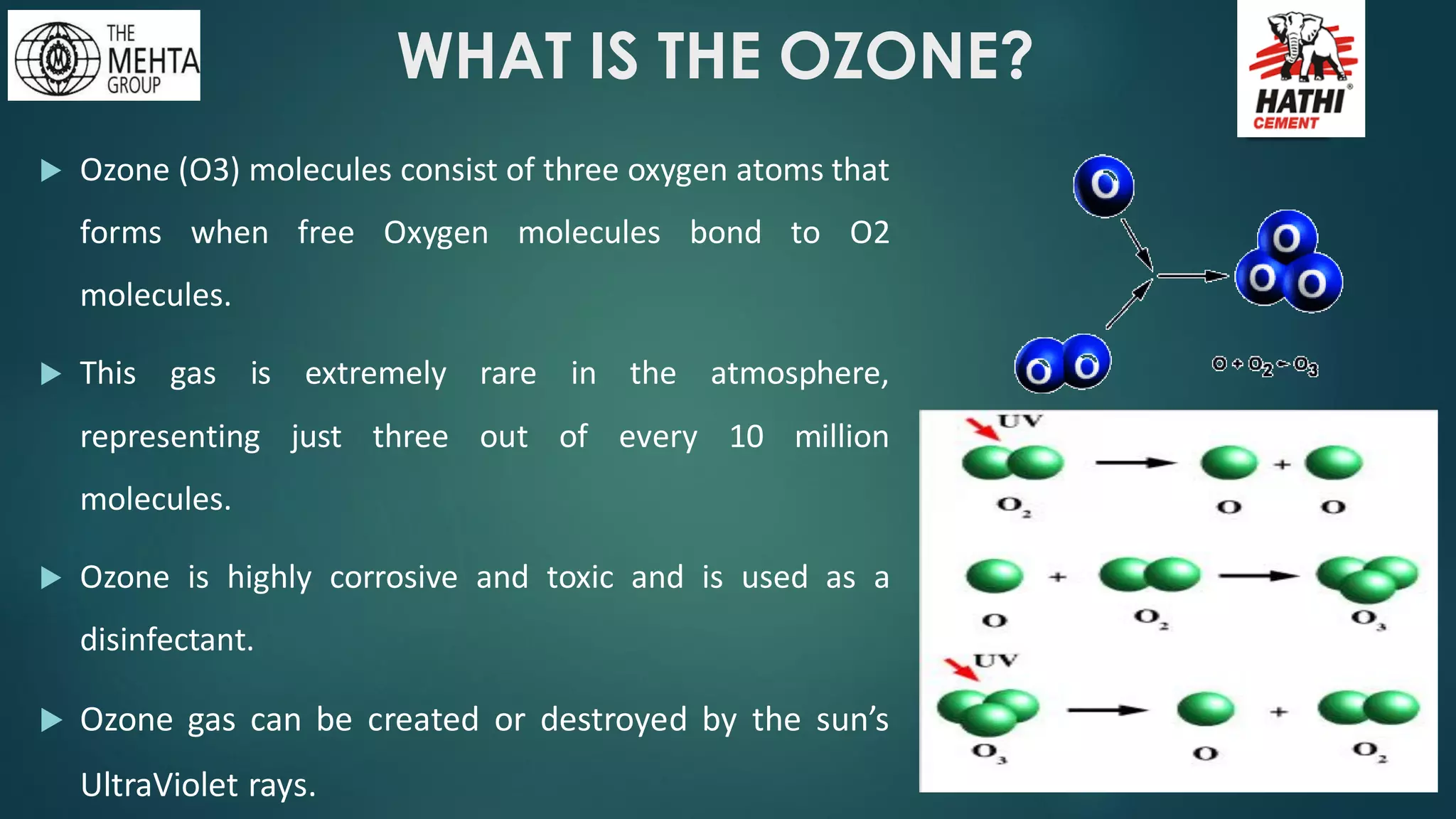
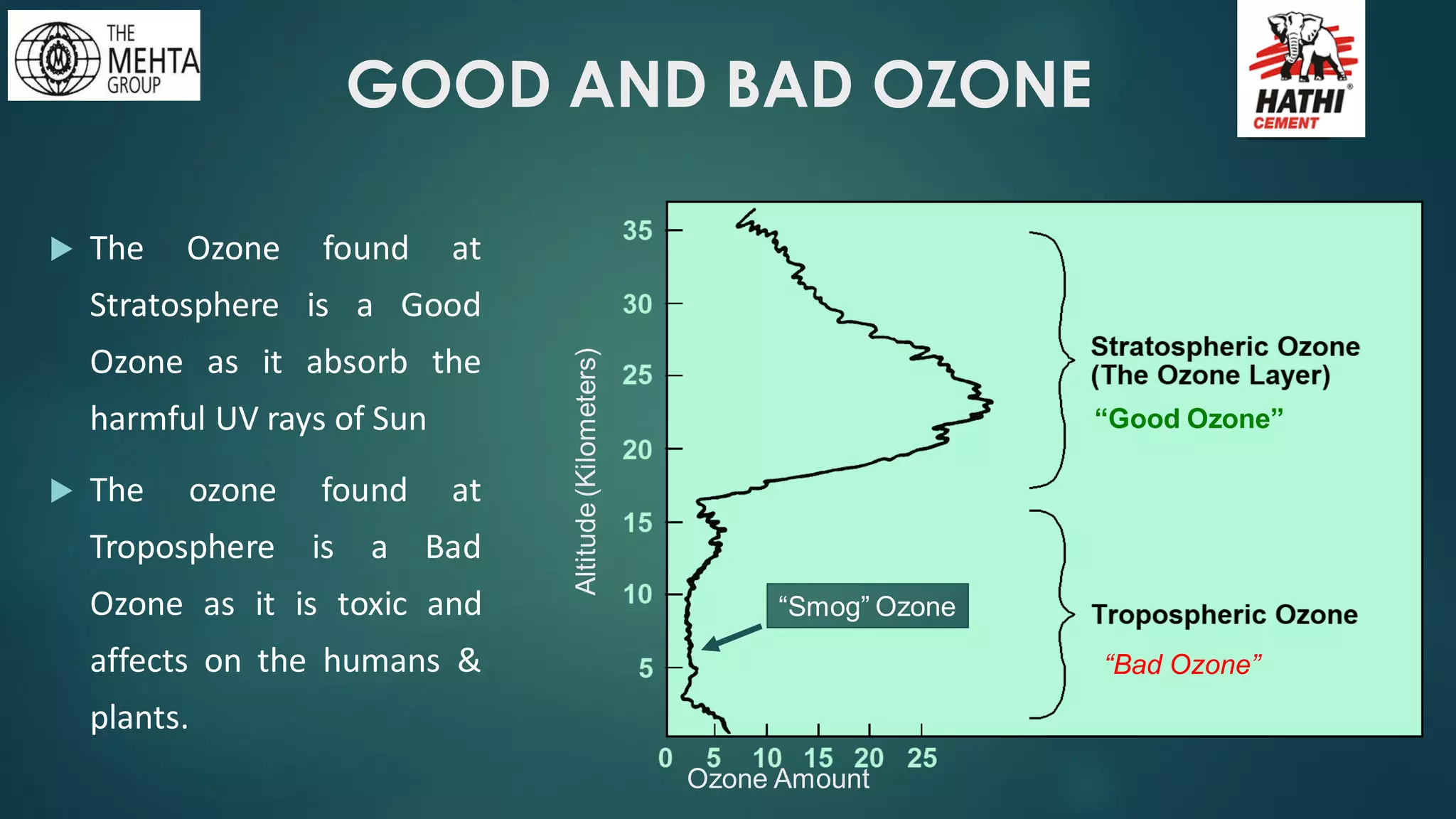
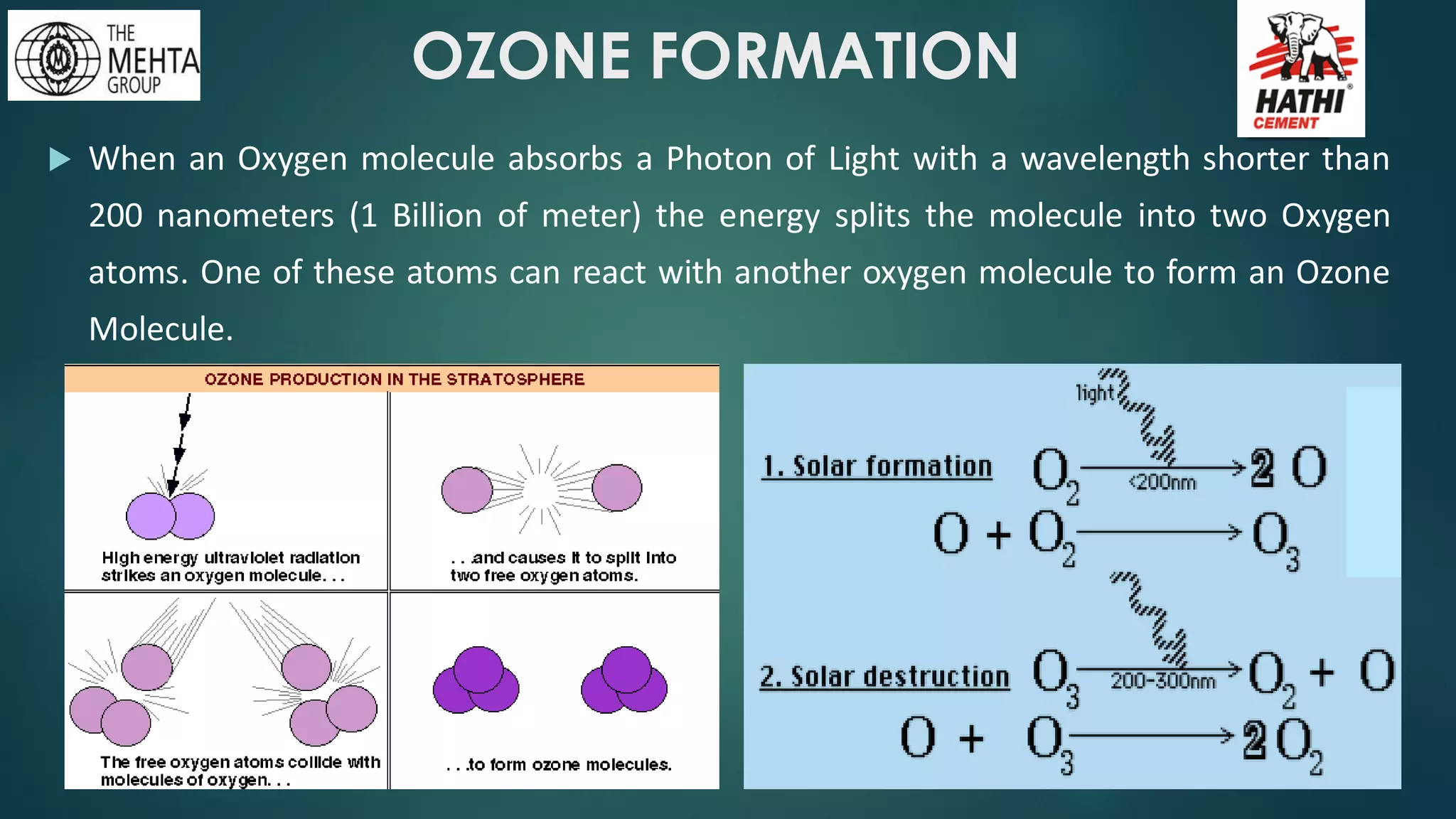
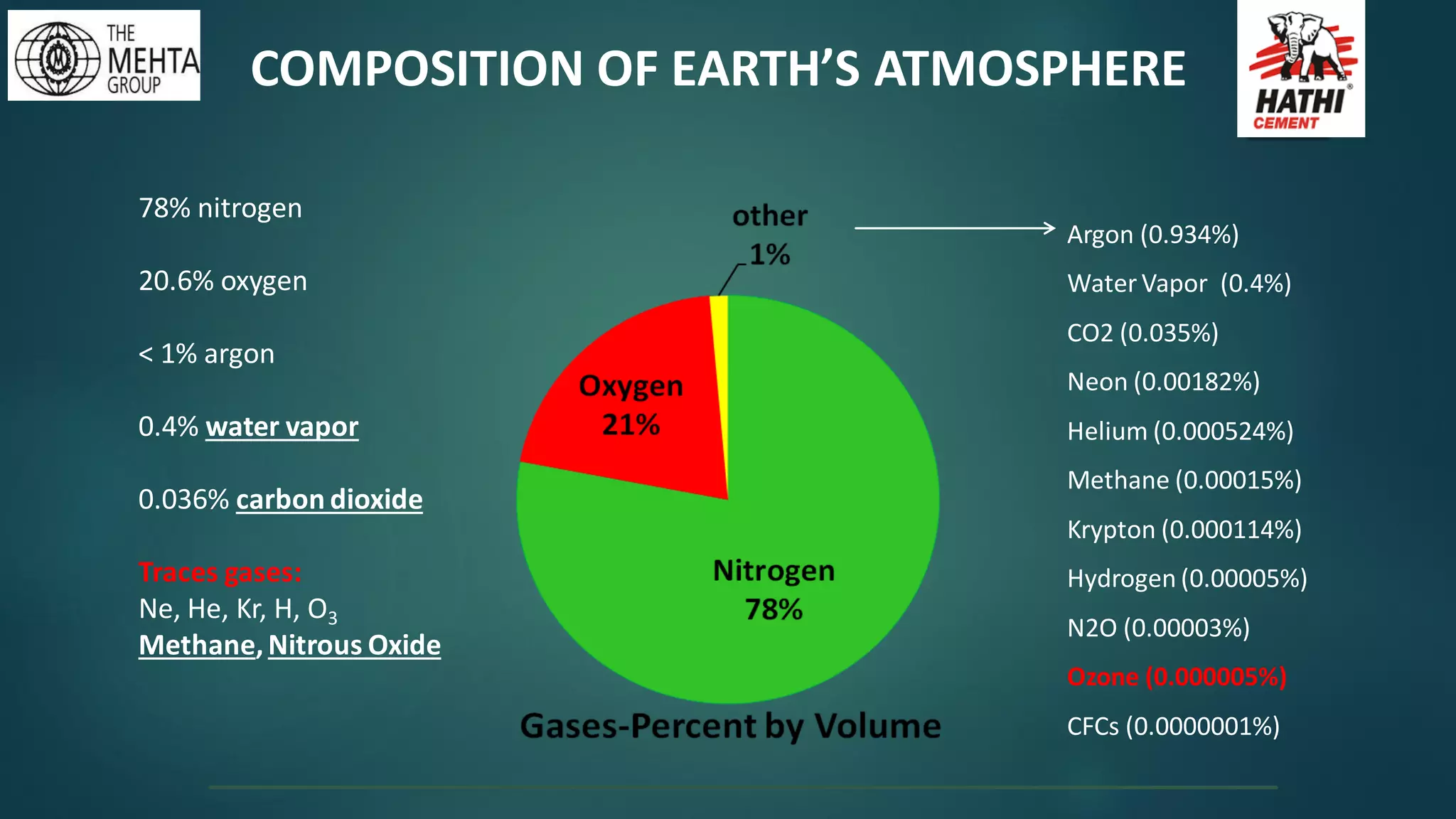
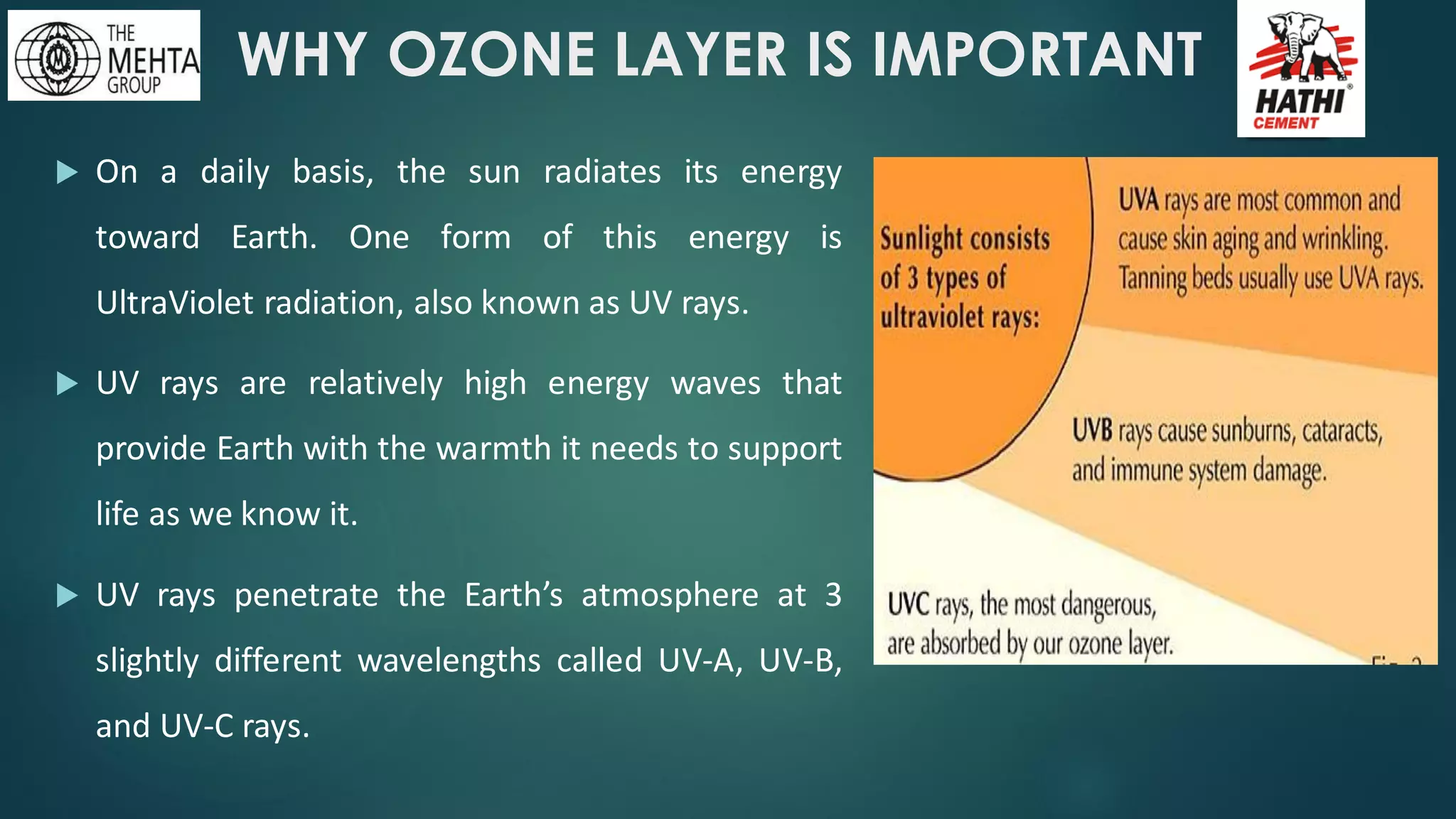
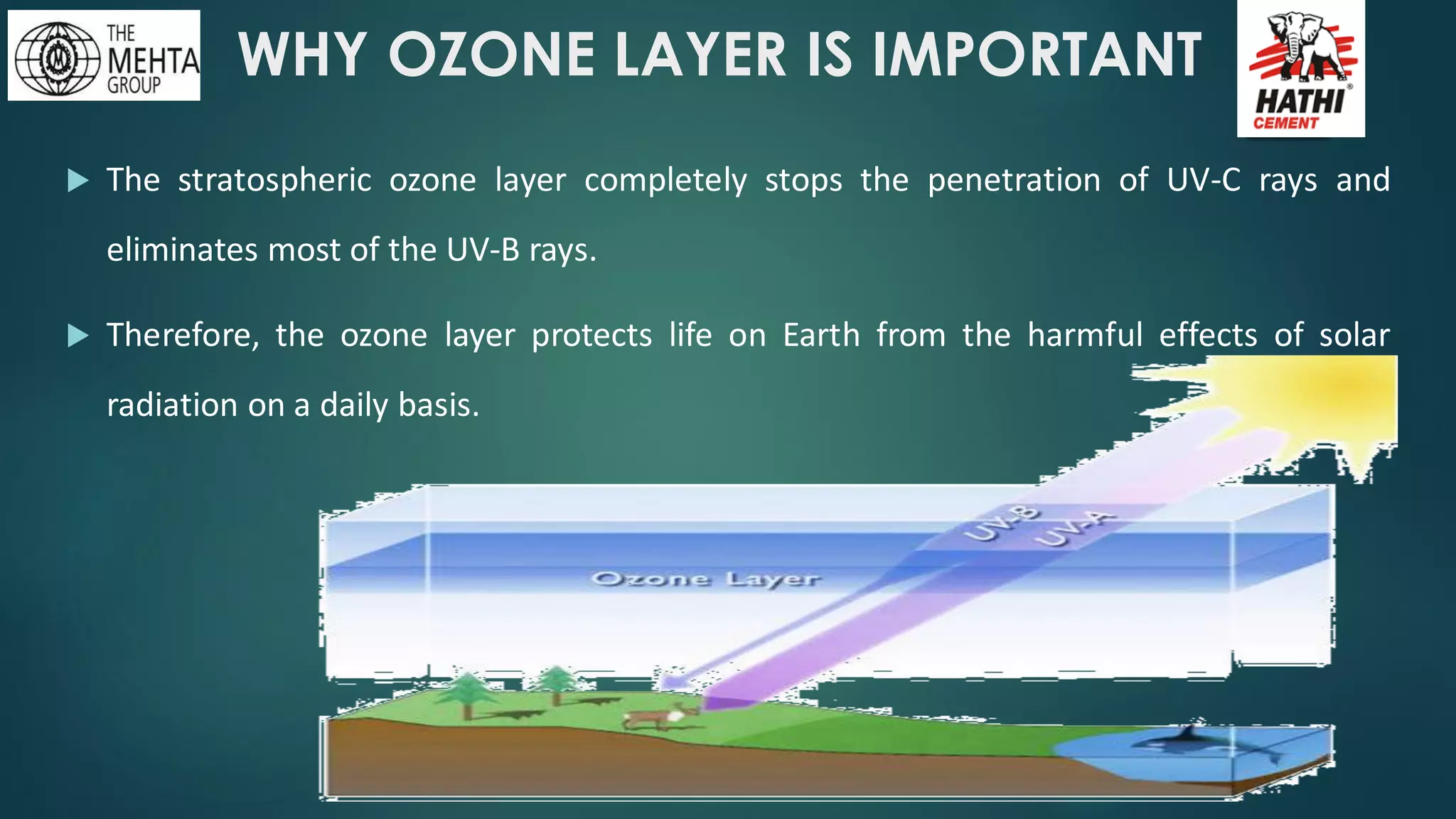
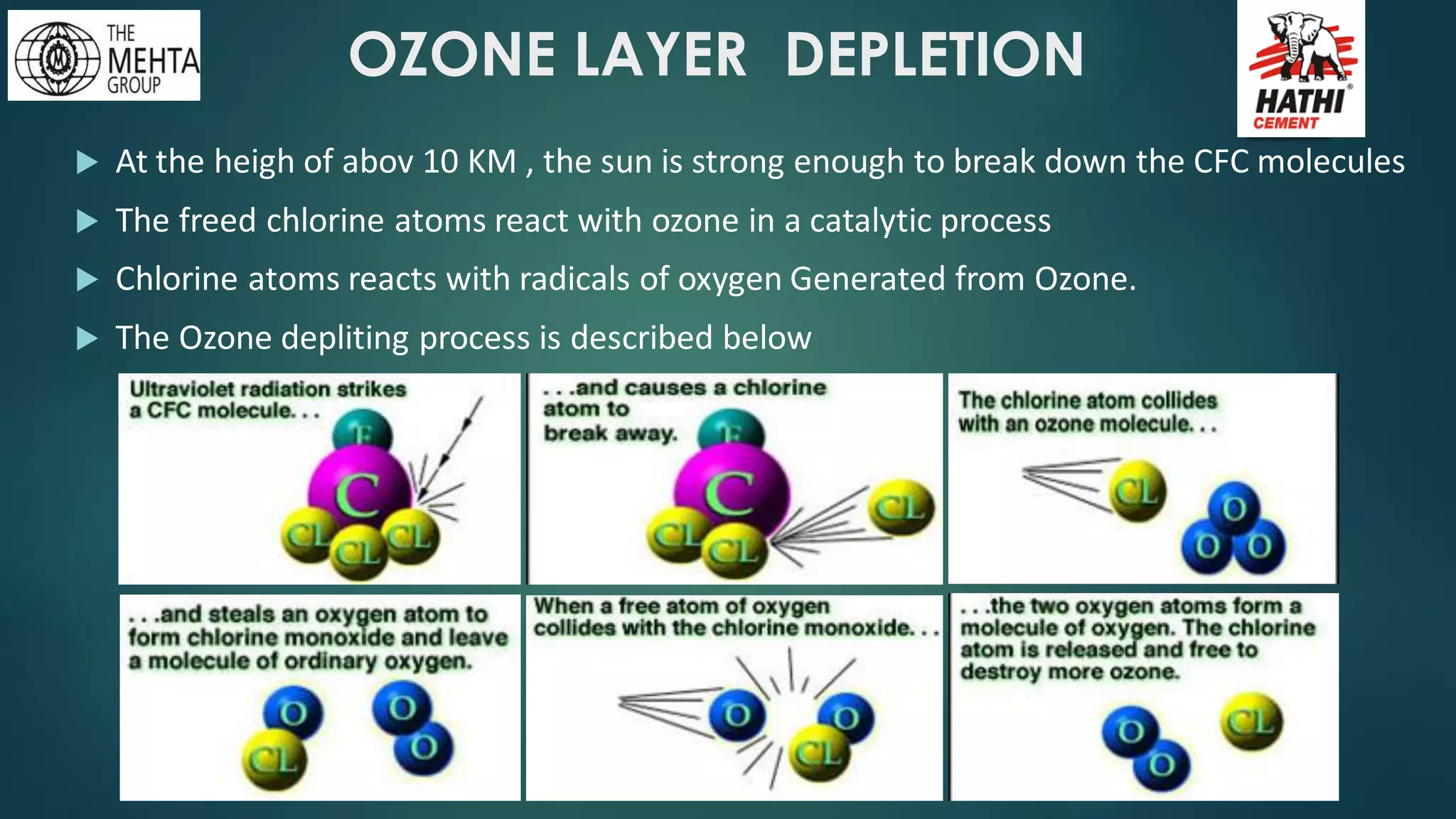
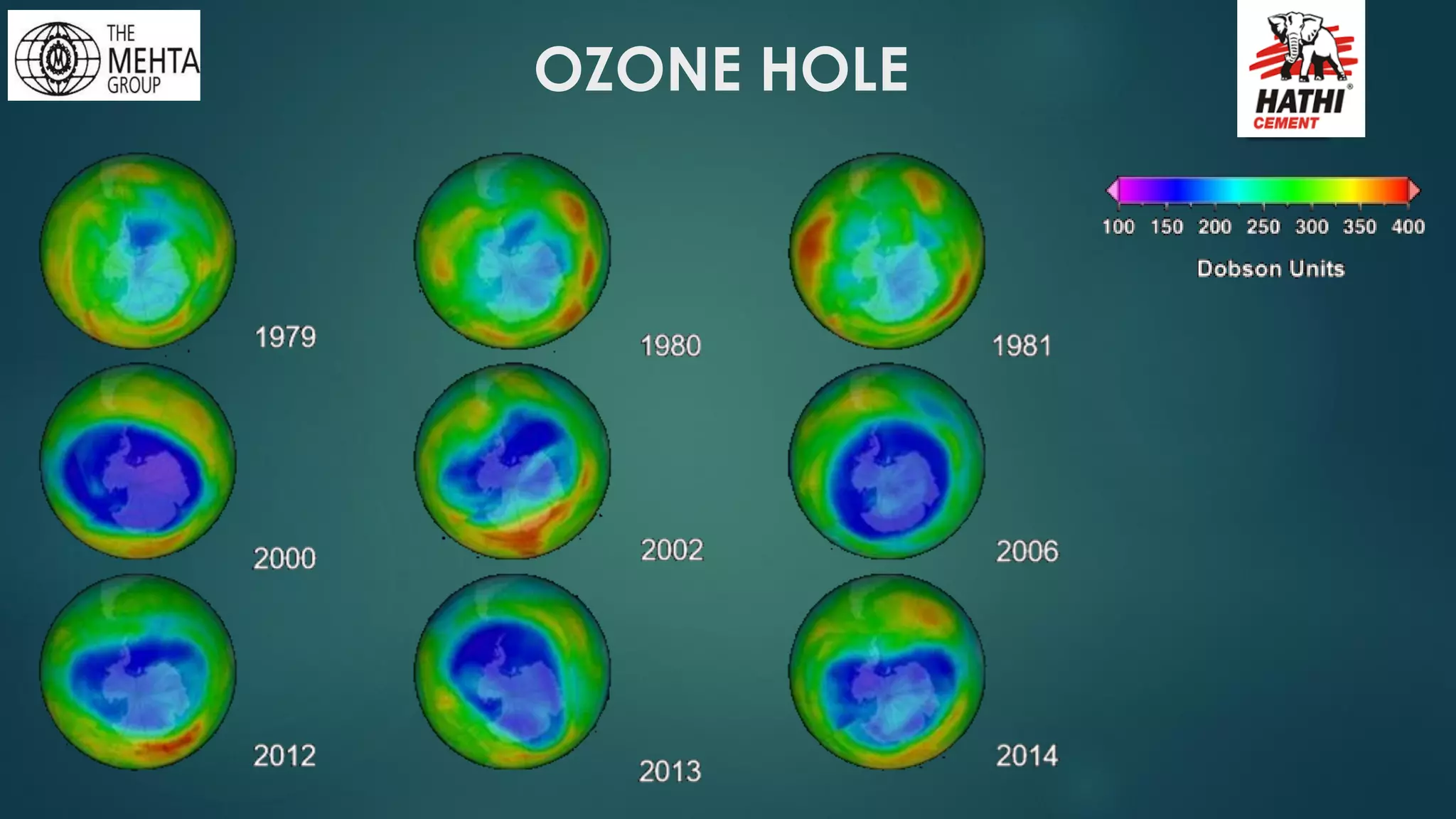
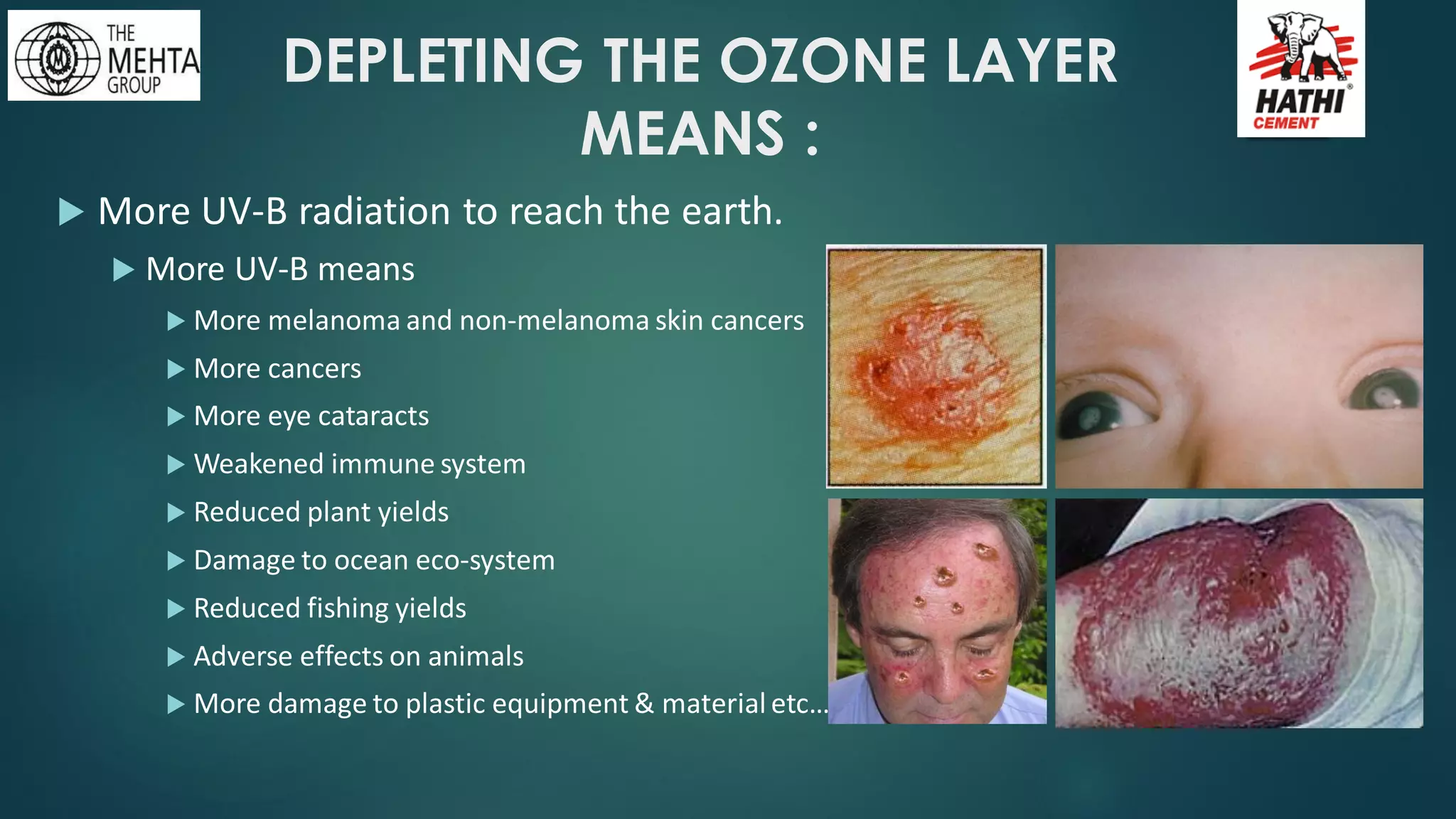
The presentation by Mr. Nikesh Banwade discusses the ozone layer, its importance in protecting life on Earth from harmful UV rays, and the detrimental effects of its depletion caused primarily by chlorofluorocarbons (CFCs). It highlights the historical context of the problem, including the discovery of the ozone hole and subsequent international efforts like the Montreal Protocol to mitigate ozone-depleting substances. The document also touches on individual actions that can contribute to ozone layer recovery and the broader environmental impact of ozone depletion.