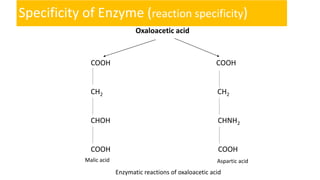
Enzymes are proteins that act as biological catalysts to speed up biochemical reactions. They are classified based on the type of reaction they catalyze and have a complex three-dimensional globular structure. Enzymes specifically bind to substrates through their active sites to lower the activation energy of reactions, speeding up the formation of products. The mechanism of enzyme action was originally proposed to be lock-and-key binding, but is now understood to involve induced fit, where the enzyme and substrate mutually adjust their shapes for catalysis. Enzymes can be regulated by various factors like substrate concentration, temperature, pH, inhibitors, and activators.