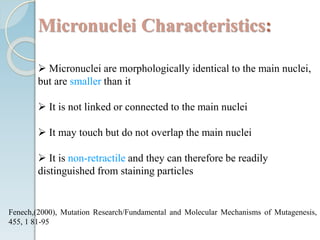
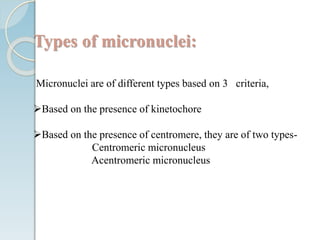
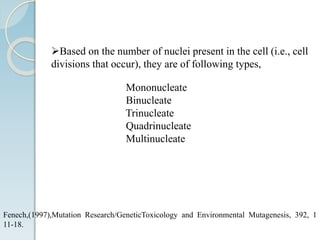
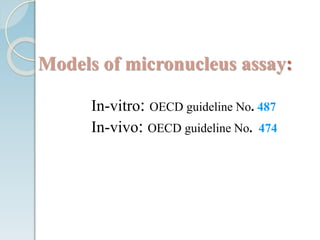
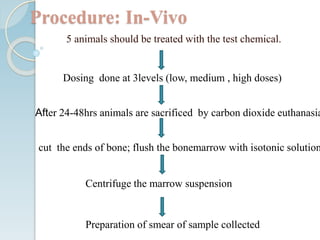
The micronucleus assay is a test used to detect potential genotoxic compounds. It works by identifying micronuclei, which form during cell division from chromosome fragments or whole chromosomes damaged by genotoxins. The assay has regulatory approval and can be conducted in vitro using cell cultures or in vivo using rodents. Cells or animals are exposed to test compounds, cell division is blocked, and cells are analyzed microscopically for the presence of micronuclei to determine if the compound caused genetic damage. The micronucleus assay is a simple, reliable, and reproducible method for toxicological testing.