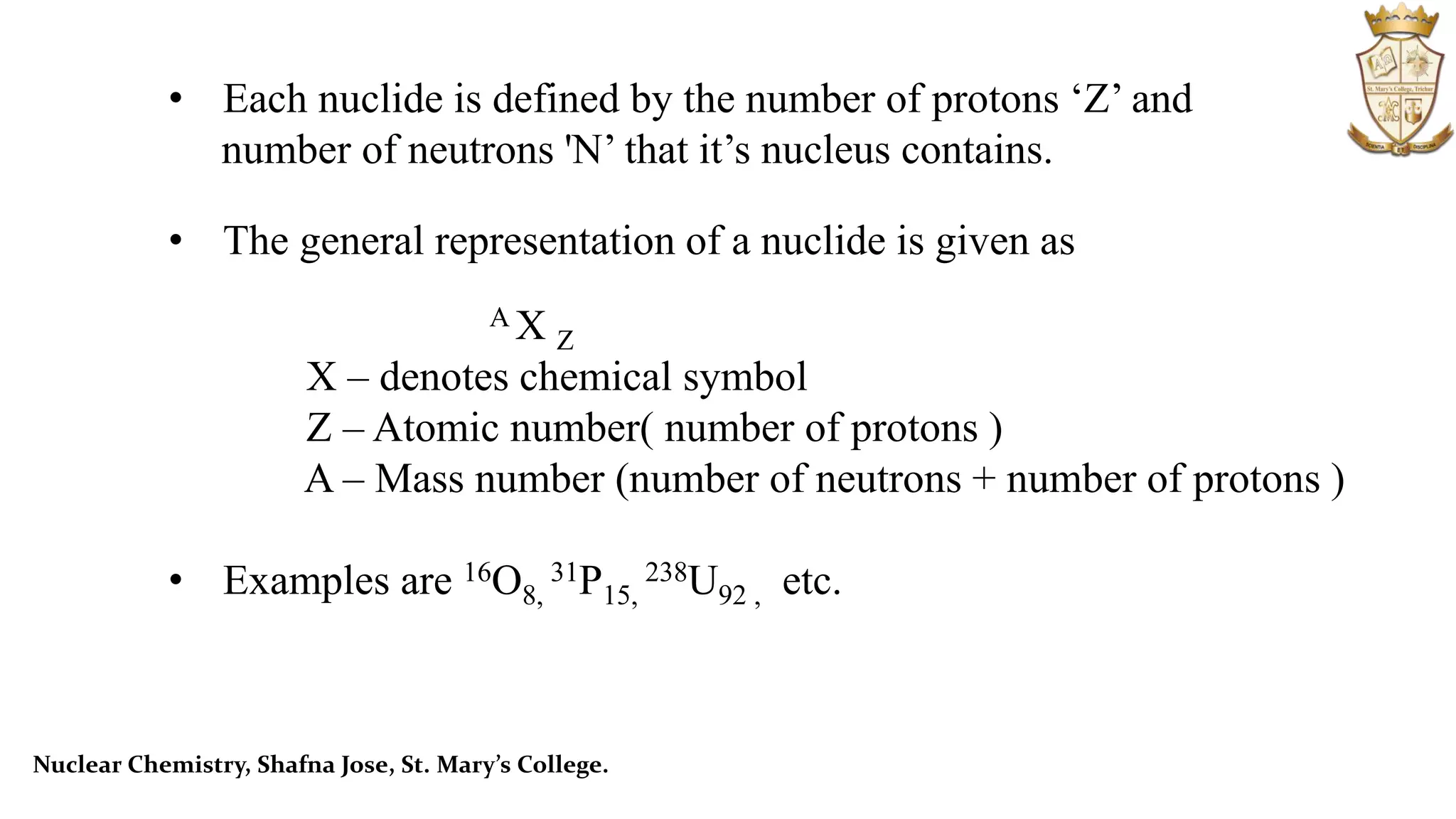
The document provides an overview of nuclear chemistry, focusing on nuclear particles, their interactions, and reactions such as radioactive decay, fission, and fusion. It explains concepts like mass defect, binding energy, and introduces models like the liquid drop model to illustrate nuclear properties. Additionally, it distinguishes between radioactivity and artificial radioactivity while discussing the energy changes in nuclear reactions.




![Mass Defect
• It is equal to the mass lost as an equivalent amount of energy during the formation of a given nucleus
from the component nucleons.
• If M, mp and mn are the masses of the nucleus (AXZ), a proton and a neutron, respectively, then
∆m = Zmp + Nmn – M = Zmp + (A – Z)mn - M
Binding Energy
• Energy released during the formation of a nucleus from it’s constituent nucleons.
• If ∆m is the mass defect , then binding energy , B.E = ∆mc2 .
B.E = [ Zmp + (A – Z)mn – M]c2
Nuclear Chemistry, Shafna Jose, St. Mary’s College.](https://image.slidesharecdn.com/nuclearchemistry-190626044514/75/Chemisrty-Nuclear-chemistry-6-2048.jpg)








