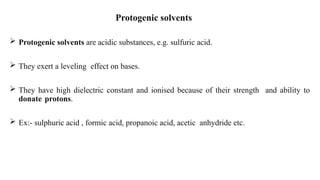
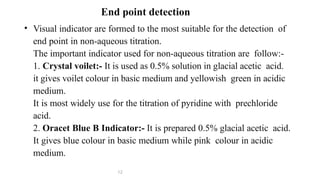
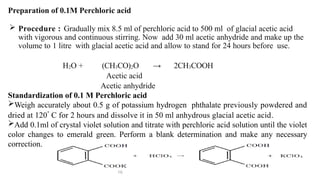
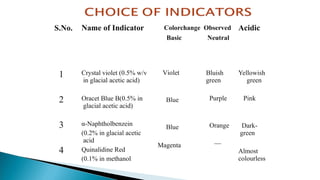
Non-aqueous titrations involve the titration of substances in solvents other than water, commonly used in pharmacopoeial assays. They are suitable for titrating very weak acids and bases, using various types of solvents like aprotic, protogenic, protophilic, and amphiprotic. The document details the selection of solvents, titration methods, indicators, and advantages of non-aqueous titrations, highlighting their applications in accurately determining substances that are insoluble or poorly soluble in water.