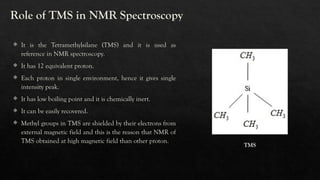
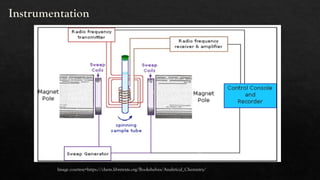
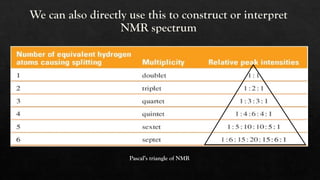
Nuclear Magnetic Resonance (NMR) spectroscopy is a vital analytical technique for determining the molecular structure of compounds by measuring interactions of nuclear spins in a magnetic field. Tetra-methylsilane (TMS) serves as the reference compound due to its unique properties, allowing for precise chemical shift measurements. NMR is widely applied in organic chemistry, medical imaging, and drug analysis, facilitating rapid non-destructive chemical analyses.