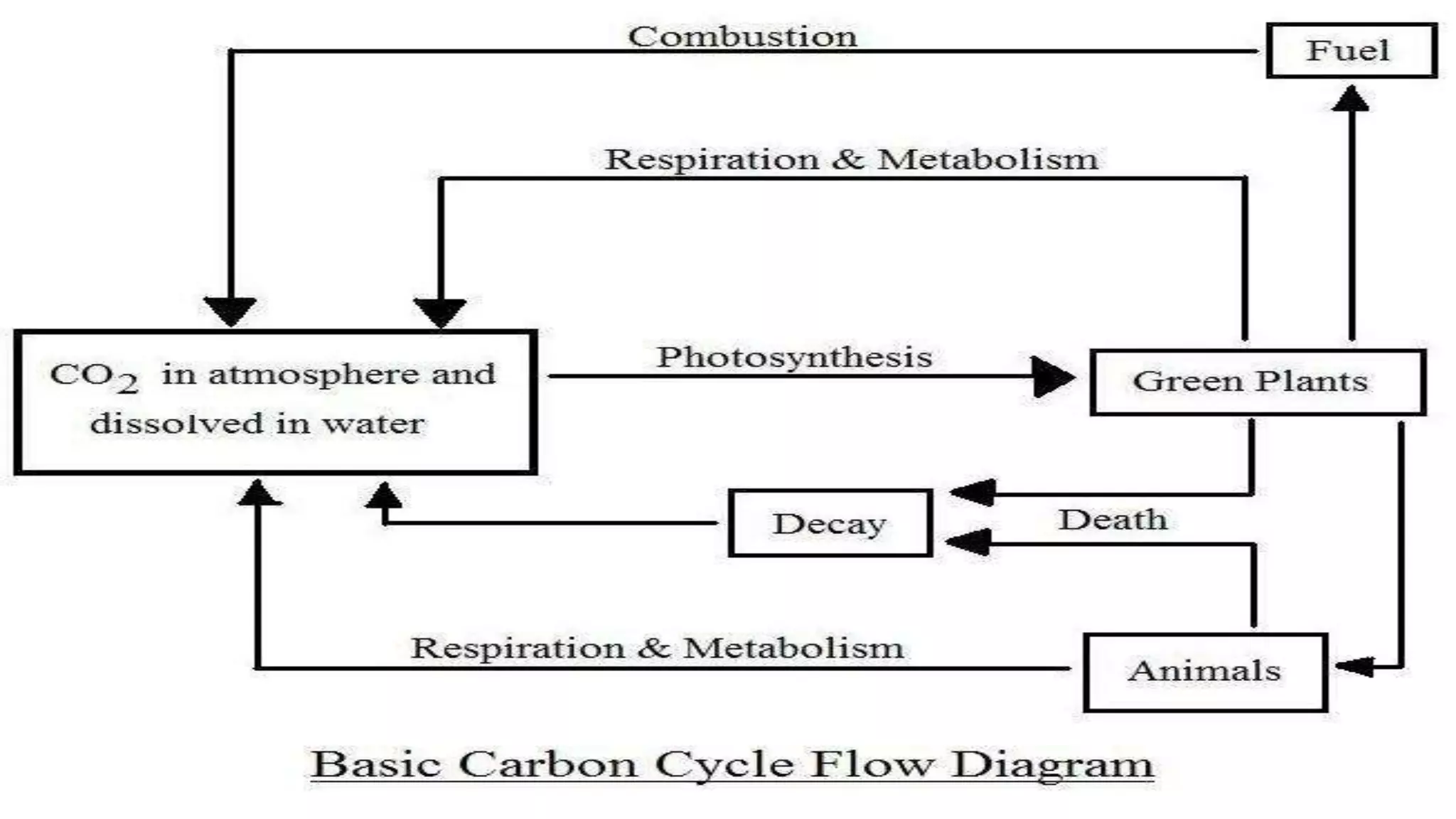
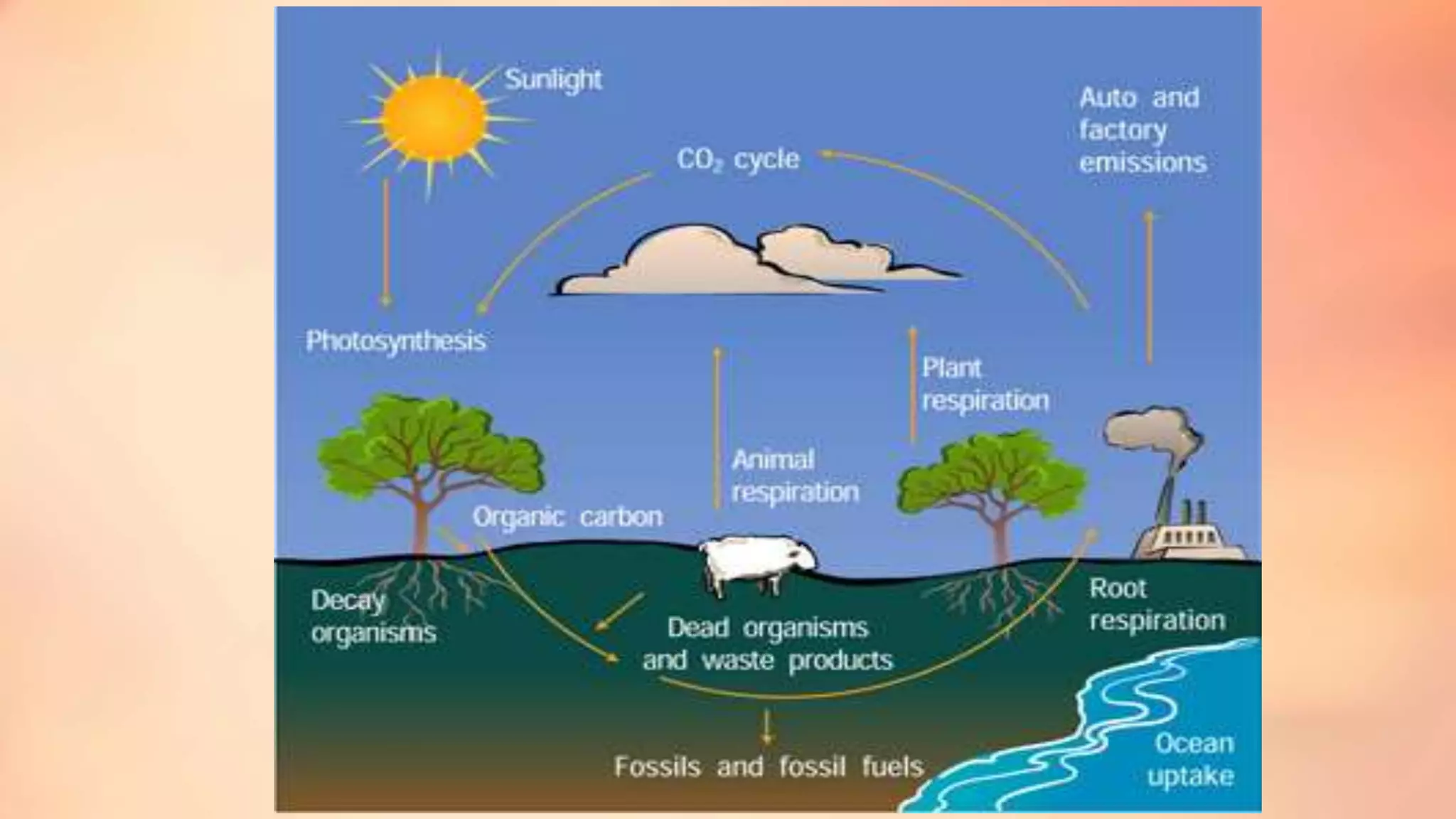
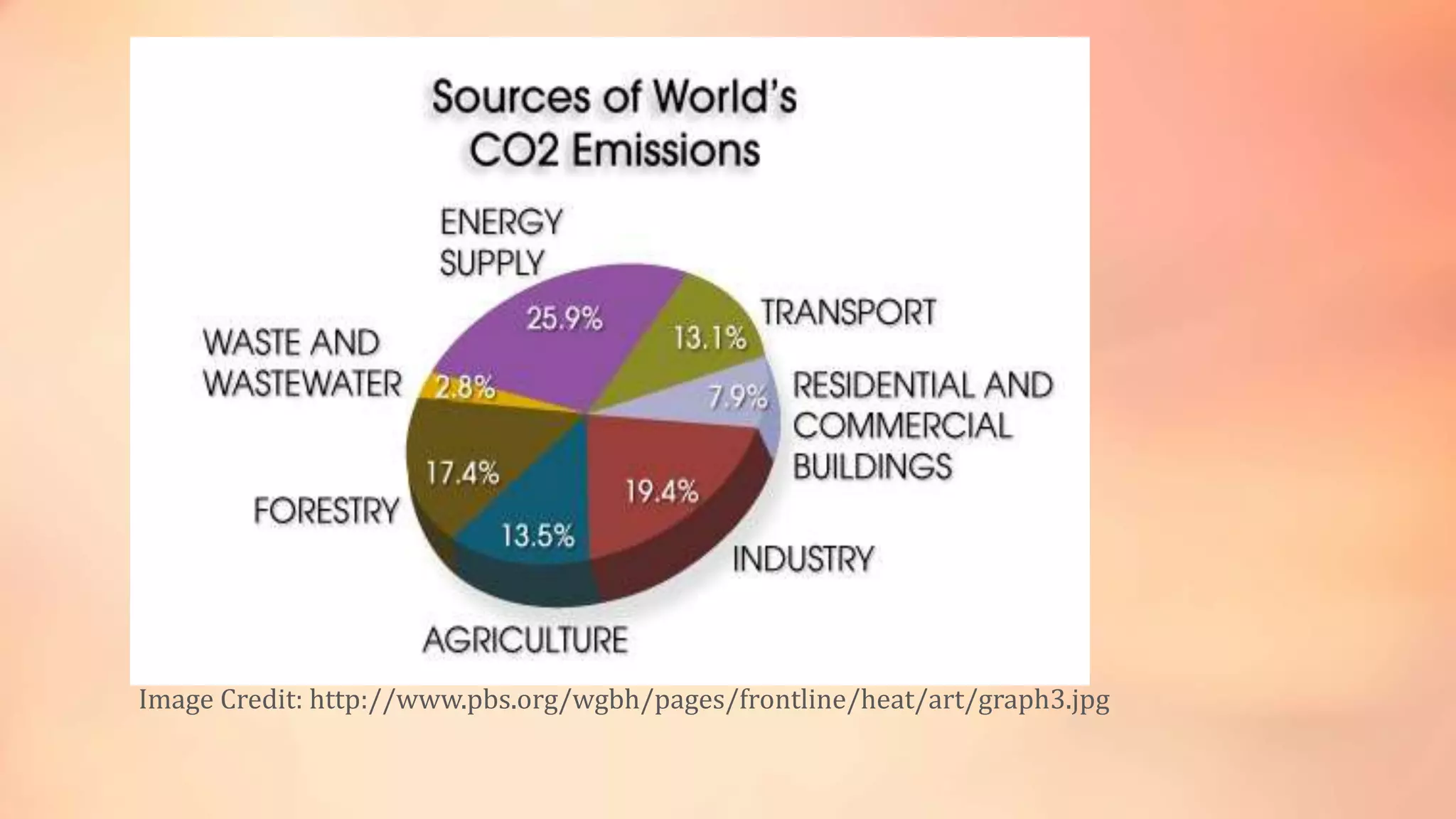
The carbon cycle describes the movement of carbon through the biosphere, geosphere, hydrosphere and atmosphere. Carbon is exchanged through processes like photosynthesis, respiration, and the weathering of rocks. Recently, the carbon cycle has become imbalanced due to human activities like burning fossil fuels and deforestation, which emit carbon dioxide into the atmosphere faster than natural processes can reabsorb it. The excess carbon dioxide is causing global warming and has negative effects on plants, humans and the climate.