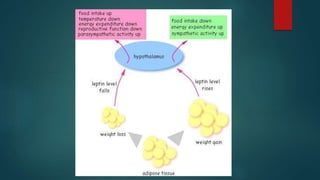
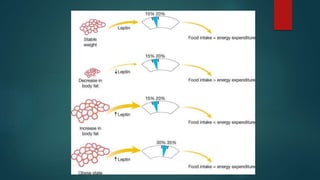
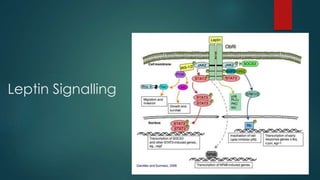
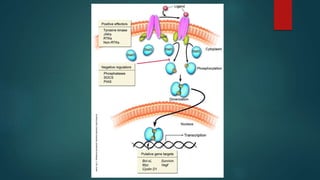
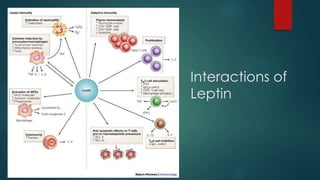
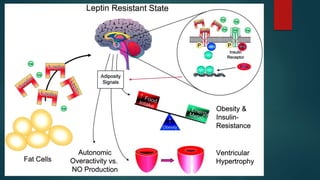
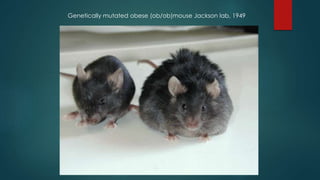
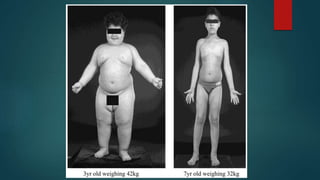
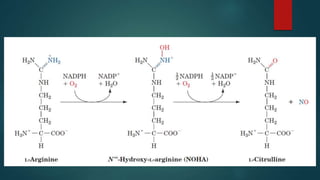
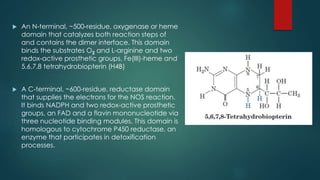
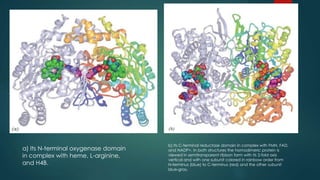
This document discusses leptin, a hormone produced by adipose tissue that signals satiety and correlates with body fat levels, and nitric oxide (NO), a reactive gas that serves as a signaling molecule in vasodilation and immune response. It explains the synthesis of NO by nitric oxide synthases (NOS) and its roles in various physiological processes, including cardiovascular health, and potential clinical applications. The document highlights how both leptin and nitric oxide can affect various health conditions, including obesity, cardiovascular diseases, and cancer.








![eNOS and nNOS but Not iNOS Are
Regulated by [Ca2+]
Ca2+–calmodulin activates eNOS and nNOS by binding to the ~30-
residue segments linking their oxygenase and reductase domains.
NO functions to transduce hormonally induced increases in
intracellular [Ca2+] in endothelial cells to increased rates of
production of cGMP in neighboring smooth muscle cells.
NO produced by nNOS mediates vasodilation through endothelium-independent
neural stimulation of smooth muscle. In this signal
transduction pathway, which is responsible for the dilation of
cerebral and other arteries as well as penile erection, nerve impulses
cause an increased [Ca2+] in nerve terminals, thereby stimulating
neuronal NOS.](https://image.slidesharecdn.com/leptinandnitricoxide-141008014903-conversion-gate01/85/Leptin-and-Nitric-Oxide-20-320.jpg)



