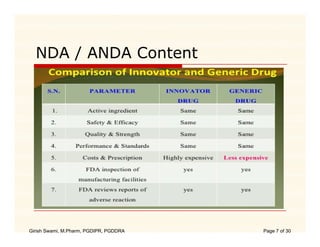
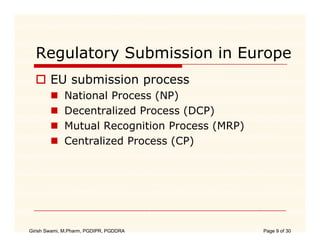
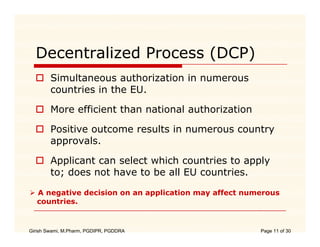
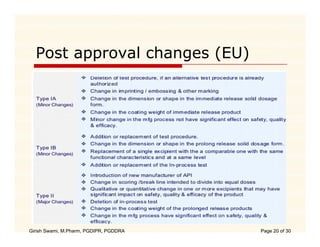
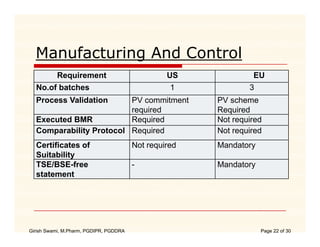
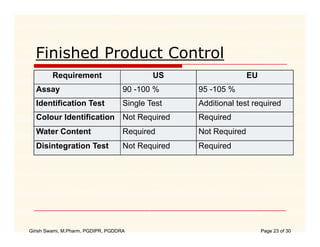
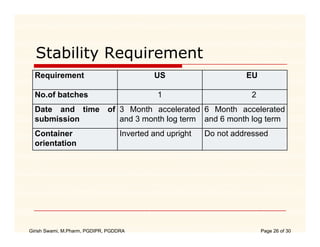
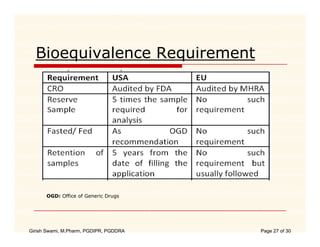
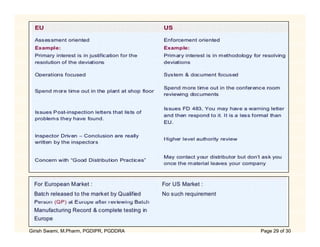
The document compares the regulatory processes for drug product submissions in the US and EU. In the US, applications are submitted to the FDA's Center for Drug Evaluation and Research and can be New Drug Applications or Abbreviated New Drug Applications. In the EU, applications are submitted through national regulatory authorities or through the centralized European Medicines Agency process. The key differences between the US and EU processes include differences in application types, approval timelines, post-approval change requirements, manufacturing standards, quality testing standards, and facility inspection processes.