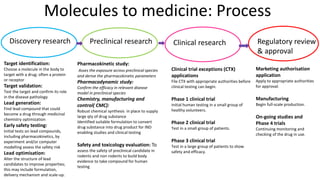
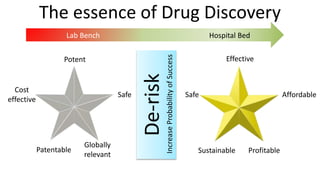
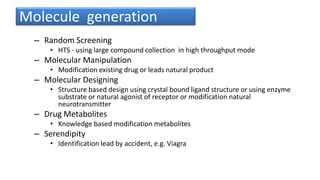
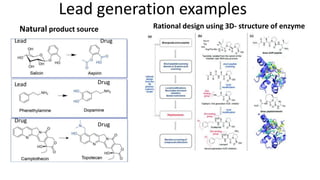
1) The process of bringing a new medicine from initial discovery to patient use (molecule to medicine) is a long, complex, and expensive process involving target identification, preclinical testing, clinical trials, and regulatory review and approval.
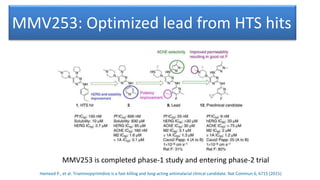
2) Preclinical testing involves evaluating a molecule's pharmacokinetics, pharmacodynamics, safety, and toxicity in cell and animal studies. Positive preclinical results allow filing an Investigational New Drug (IND) application to begin human clinical trials.
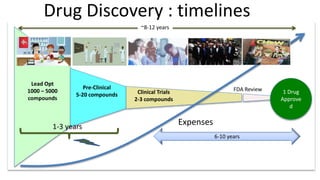
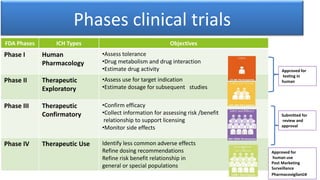
3) Clinical trials are conducted in four phases to evaluate a drug's safety, efficacy, side effects, and optimal dosing in humans. The entire development process from discovery to approval takes 8-12 years and costs over $1