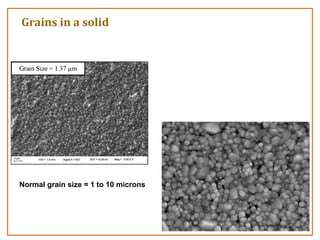
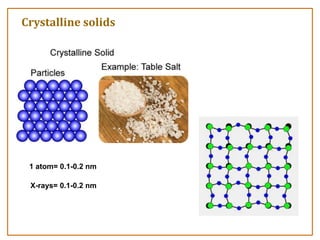
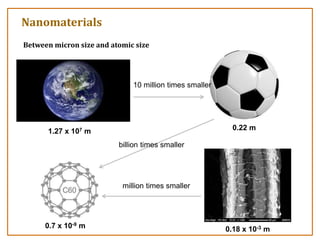
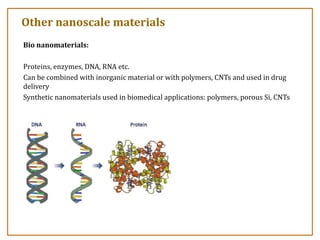
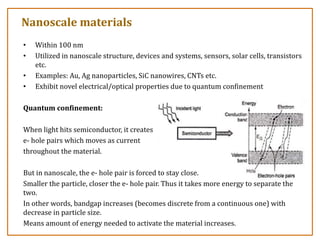
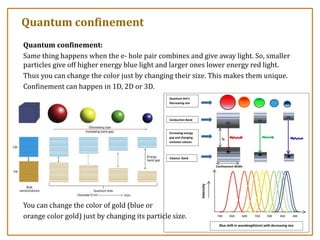
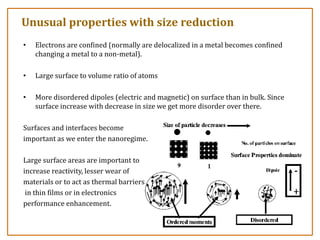
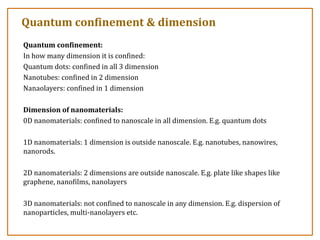
The document discusses nanosensors and nanomaterials, highlighting their size, properties, and applications in fields such as drug delivery and electronics. It explains the concept of quantum confinement, where smaller particles exhibit unique electrical and optical properties due to increased energy requirements and altered light emission characteristics. Various types of nanomaterials are categorized based on their dimensional confinement, including quantum dots, nanotubes, and nanolayers.