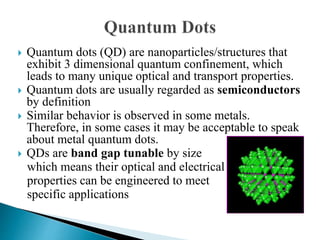
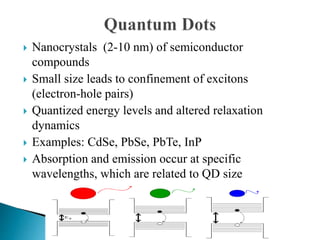
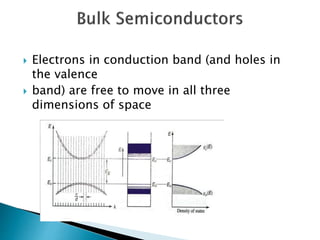
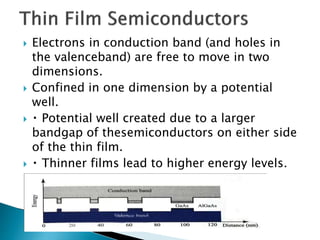
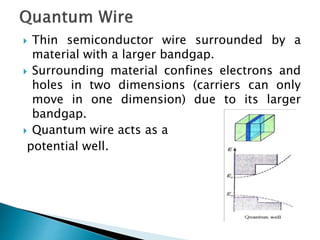
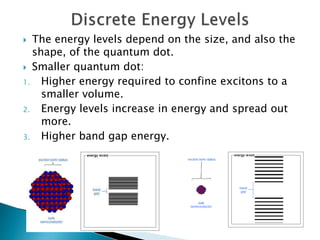
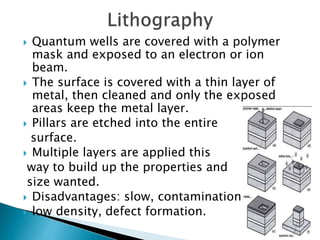
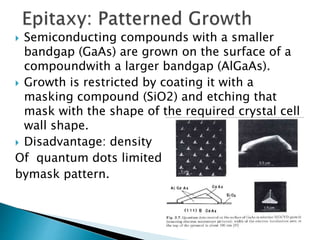
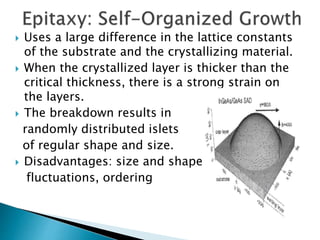
This presentation discusses quantum dots, which are nanoparticles that exhibit quantum confinement. Quantum dots are usually made of semiconductors and their optical and electrical properties depend on their size due to quantum confinement effects. They can be made through lithography, colloidal synthesis, or epitaxial growth methods. The presenter notes that quantum dots made of heavy metals like cadmium may not be commercially viable due to legislation, so silicon quantum dots are being researched as a non-toxic alternative. Potential applications of quantum dots include solar cells, biosensing, LEDs, displays, and lasers due to their size-dependent properties.