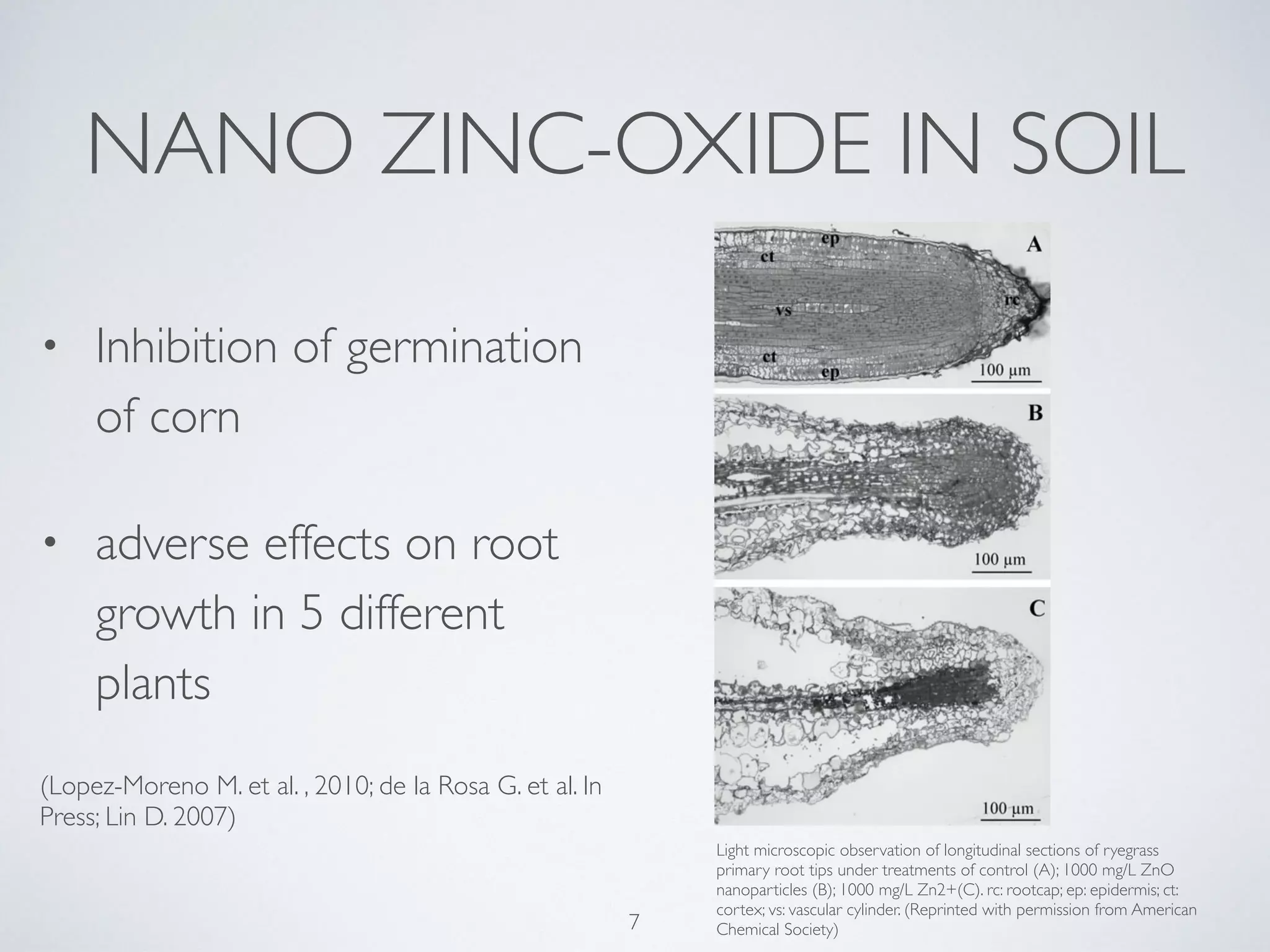
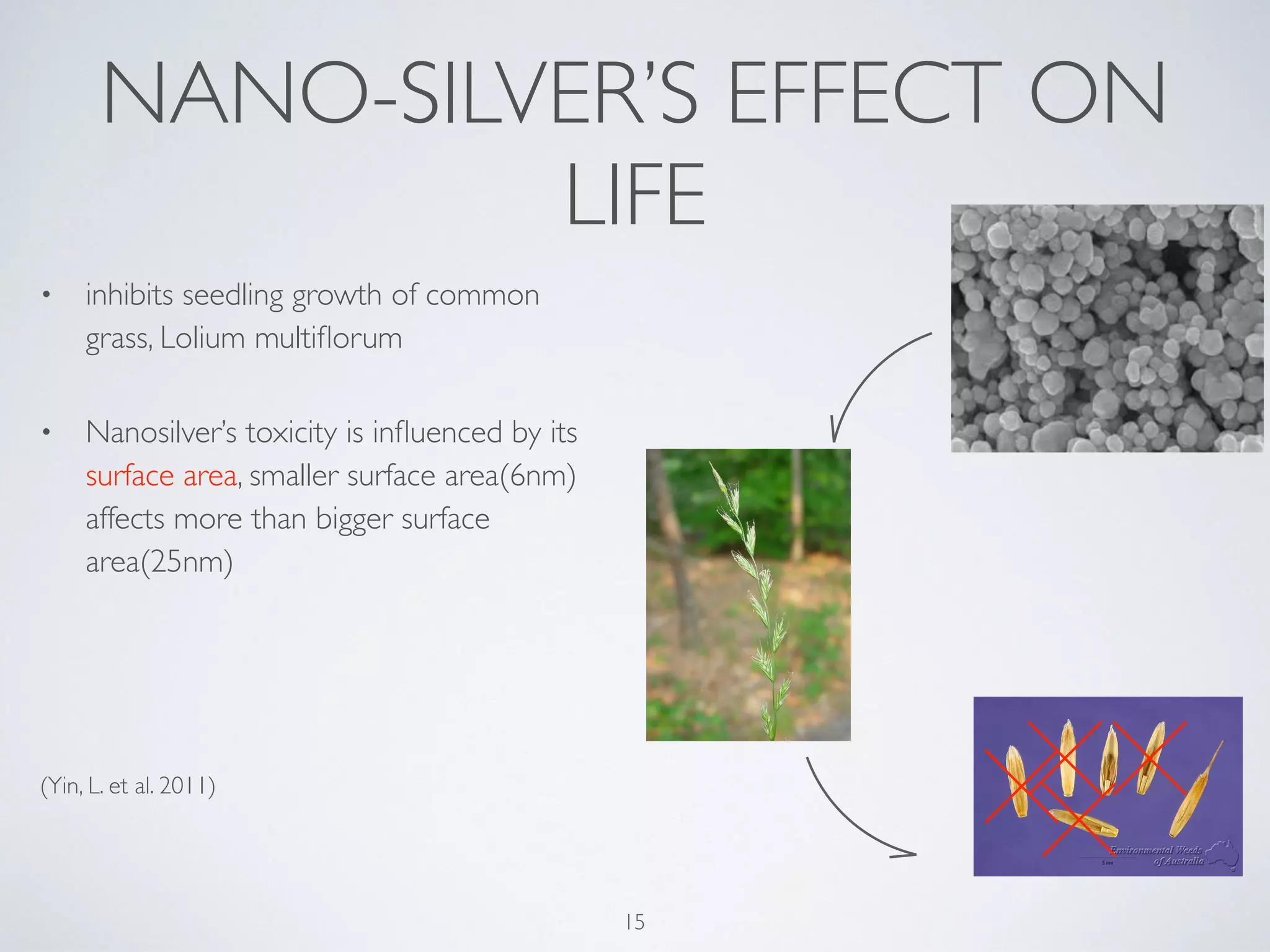
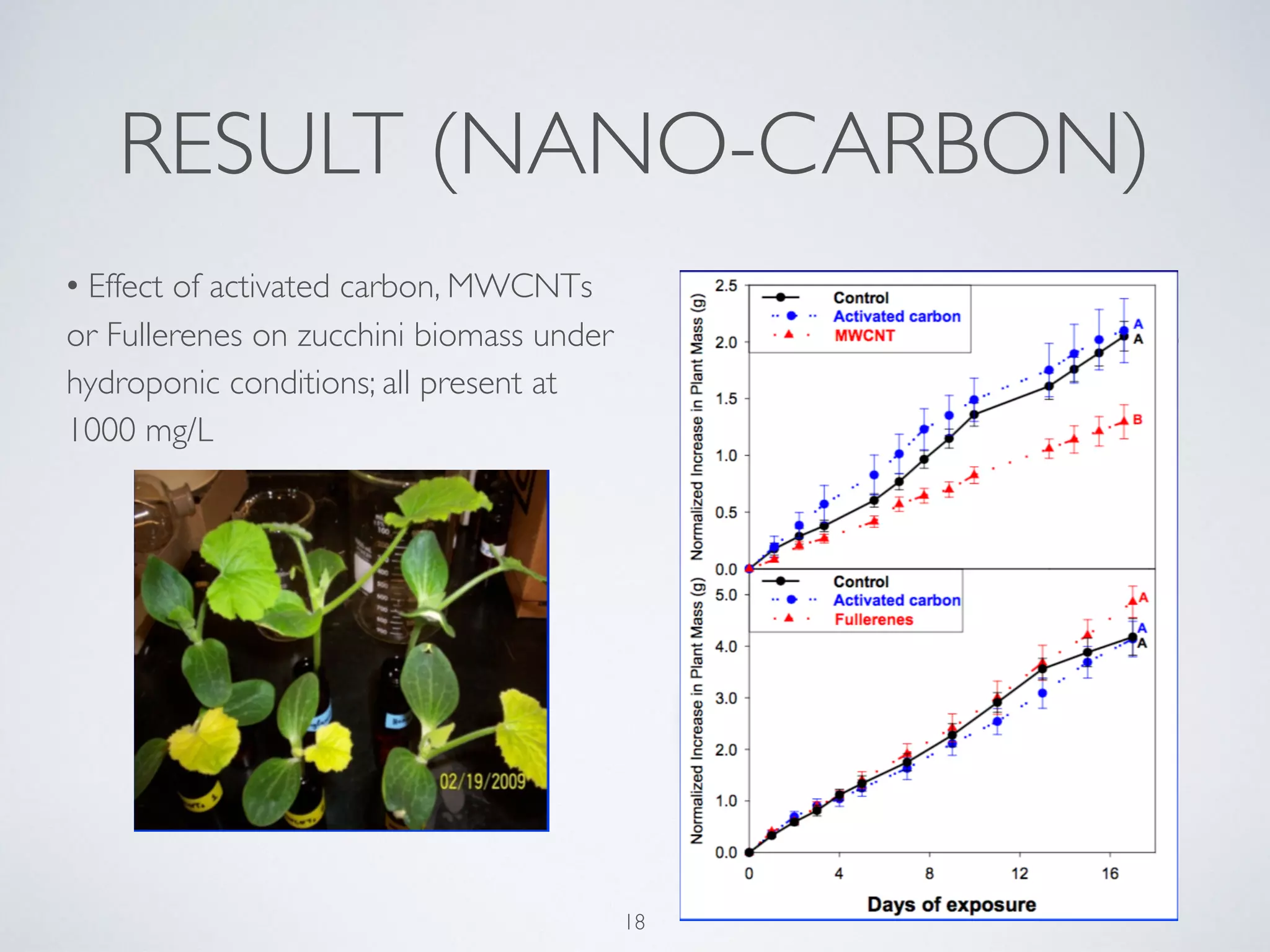
This document summarizes research on the contamination of the environment by nanoparticles. It discusses how various nanoparticles like zinc oxide, titanium dioxide, silver and silica can end up in water, soil and air. Studies show these nanoparticles can inhibit plant growth, reduce phytoplankton populations, impact aquatic life like zebrafish embryos, and reduce microbial activity in soil. Long term exposure of soil to low doses of silver nanoparticles was found to decrease the biomass of some plant species and change the soil microbe community. More research is still needed to understand the ecological effects of nanoparticle pollution and develop monitoring schemes.