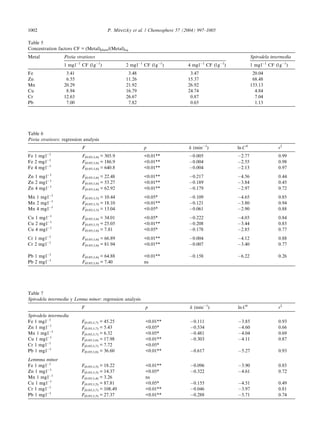
This document discusses a study that examined the potential for three species of aquatic macrophytes (Pista stratiotes, Spirodela intermedia, and Lemna minor) to simultaneously remove several heavy metals from water. In laboratory experiments, the macrophytes were exposed to varying concentrations of iron, copper, zinc, manganese, chromium, and lead over 15 days. High removal percentages for all three species and metals were observed. However, L. minor did not survive the experimental conditions. The rate of metal uptake by the macrophytes was dependent on the initial metal concentration. Overall, the study evaluated the macrophytes' ability to remove multiple heavy metals from water to help treat naturally polluted environments.