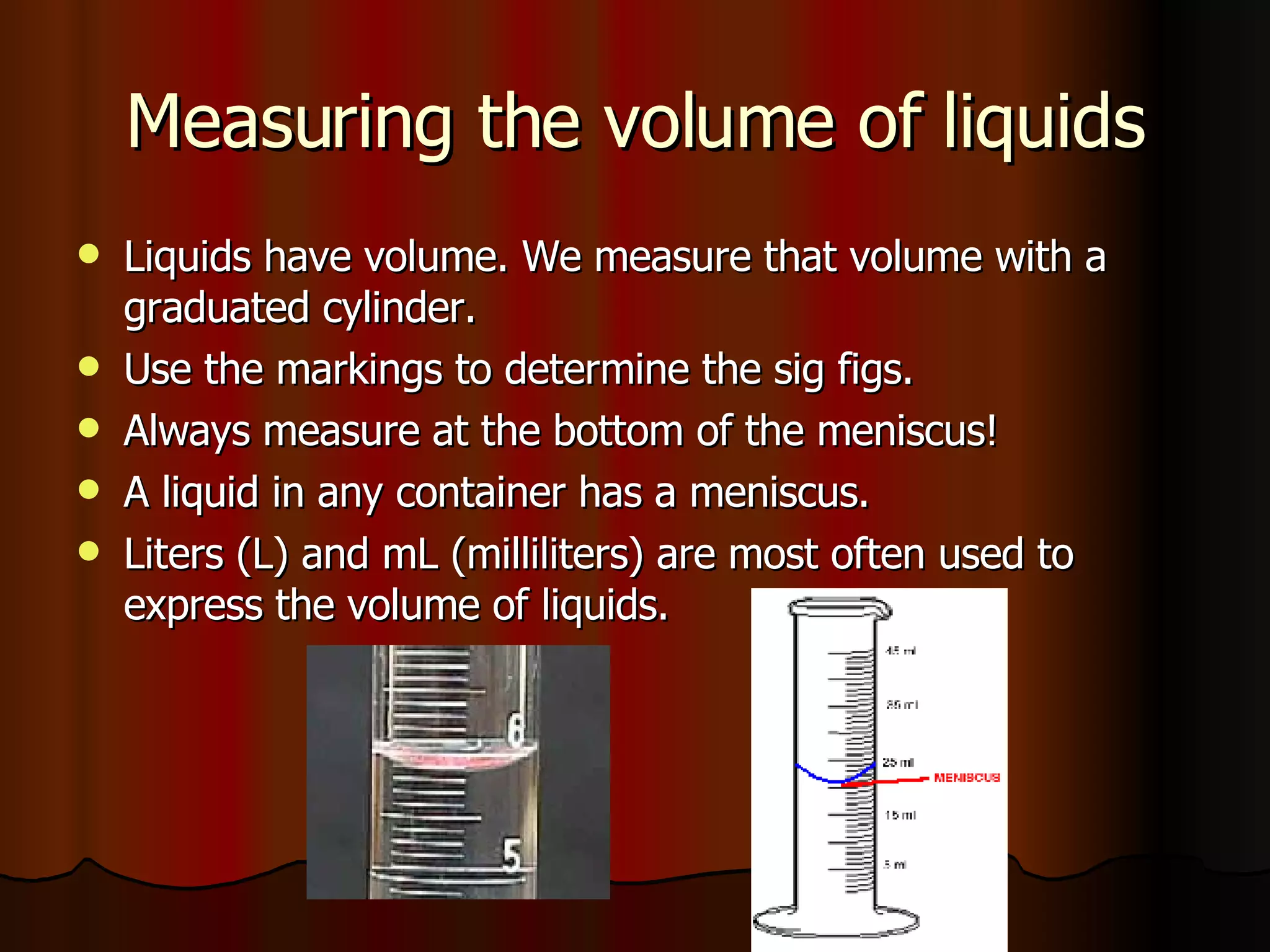
The document discusses the properties of matter including volume, mass, density, physical properties, chemical properties, and the differences between solids, liquids, and gases. It defines volume as the amount of space an object occupies, and mass as a measure of the amount of matter in an object. Density is defined as mass per unit volume. Physical properties can be observed without changing the identity of the substance, while chemical properties describe a substance's ability to change into a new substance.