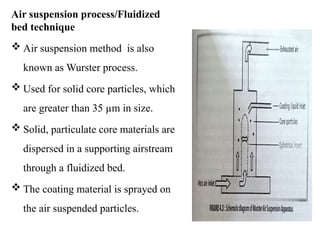
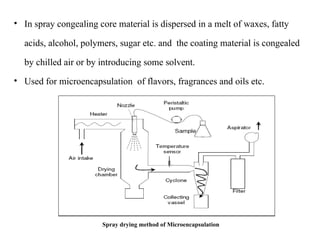
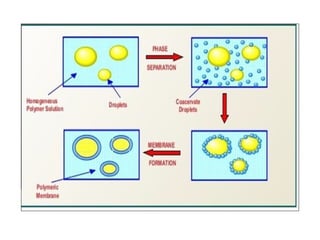
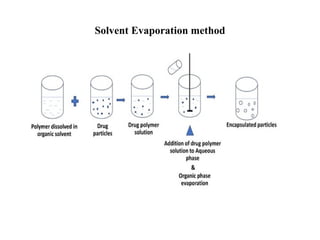
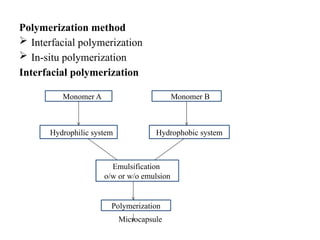
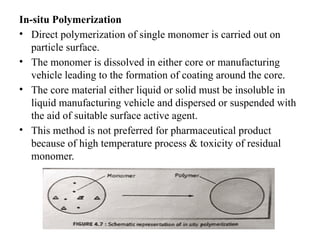
The document outlines various microencapsulation techniques including physio-mechanical, physio-chemical, and chemical methods such as air suspension, pan coating, spray drying, and coacervation. It explains processes for encapsulating solid and liquid core materials, their applications in pharmaceuticals, and the effects of different variables on the encapsulation process. Additionally, it discusses the benefits of microencapsulation, including drug protection and controlled release.