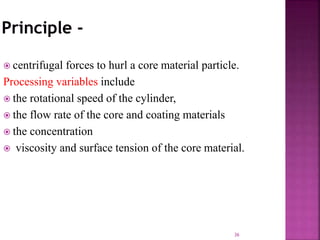
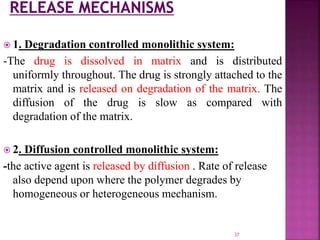
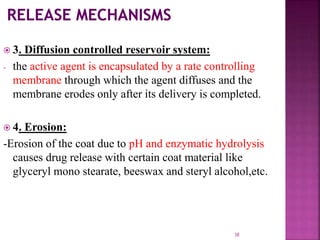
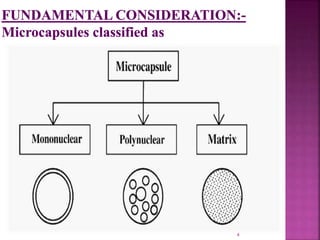
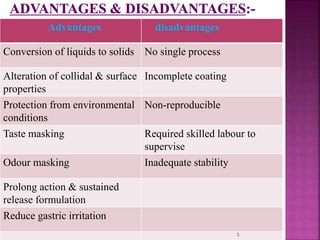
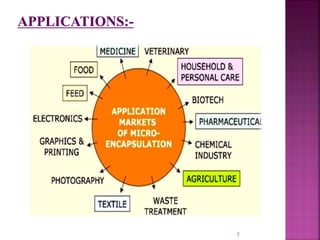
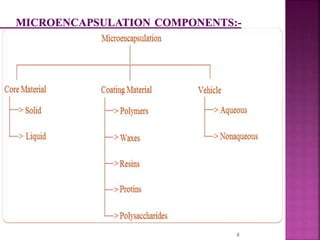
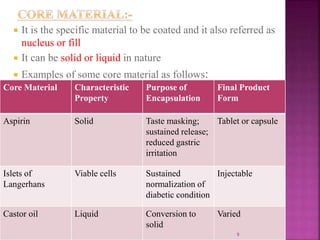
This document discusses microencapsulation. It begins by defining microencapsulation as coating small particles of solids, liquids, or gases to form microcapsules. It then discusses the core material to be coated, coating materials, and dimensions of microcapsules. Advantages and disadvantages of microencapsulation are provided. Various applications are mentioned including immobilizing bioactive compounds and protecting compounds from degradation. The key components and methods for microencapsulation are described at a high level. Finally, release mechanisms and references are briefly touched upon.








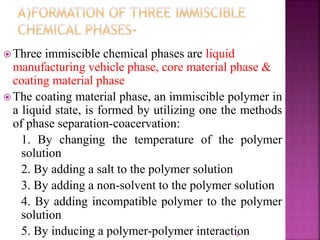
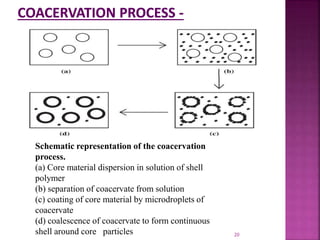
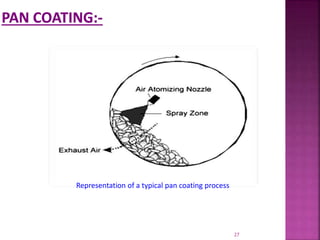
![ [1]Coacervation-phase separation:-
Coacervation means formation of aggregates to form
pdt.
Process-three steps carried out under continuous
agitation:
1. Formation of three immiscible chemical
phases
2. Deposition of the coating
3. Rigidization of the coating
17](https://image.slidesharecdn.com/microencapsulationbypravingore-190315100520/85/Microencapsulation-by-Pravin-Gore-17-320.jpg)