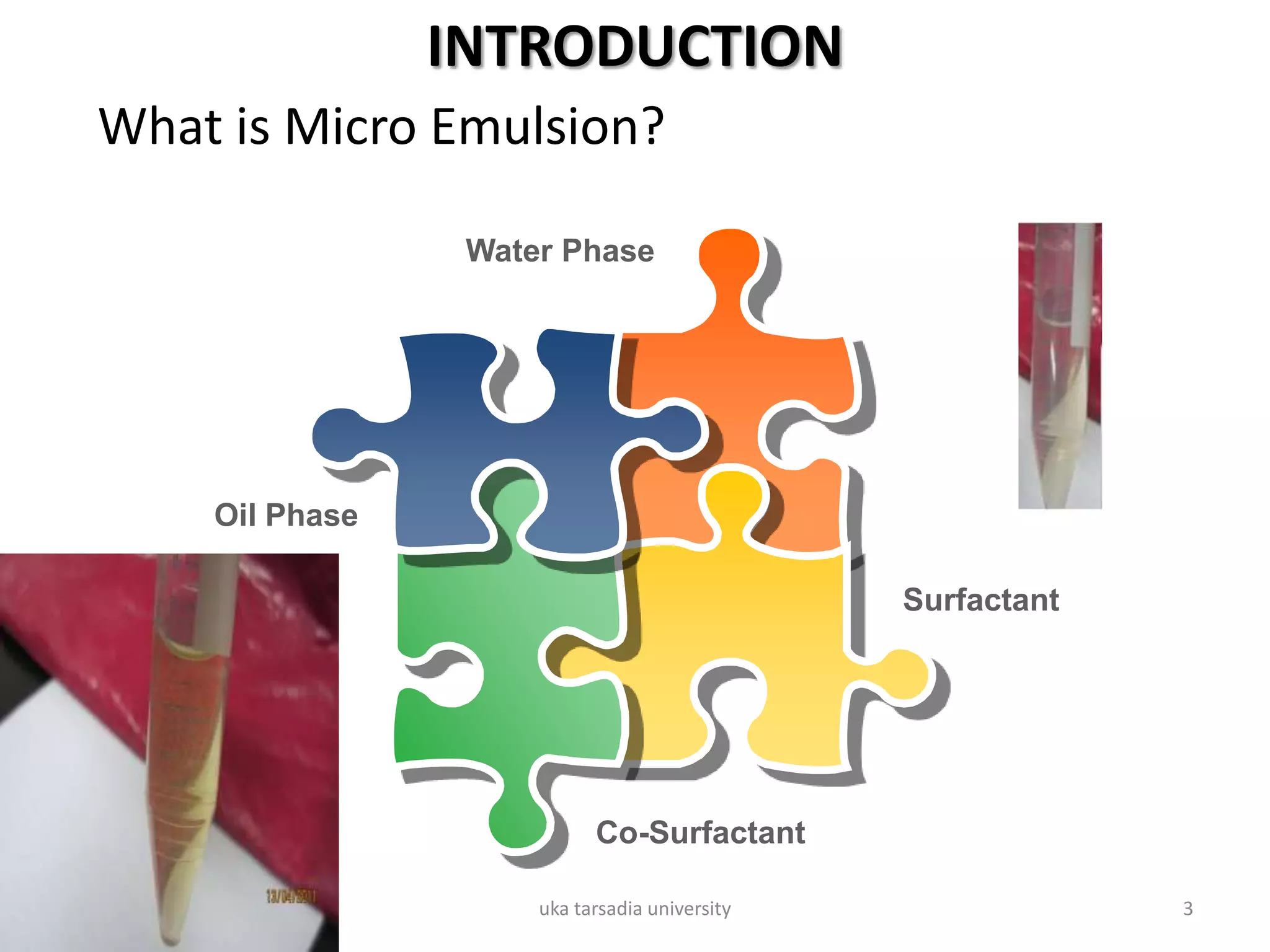
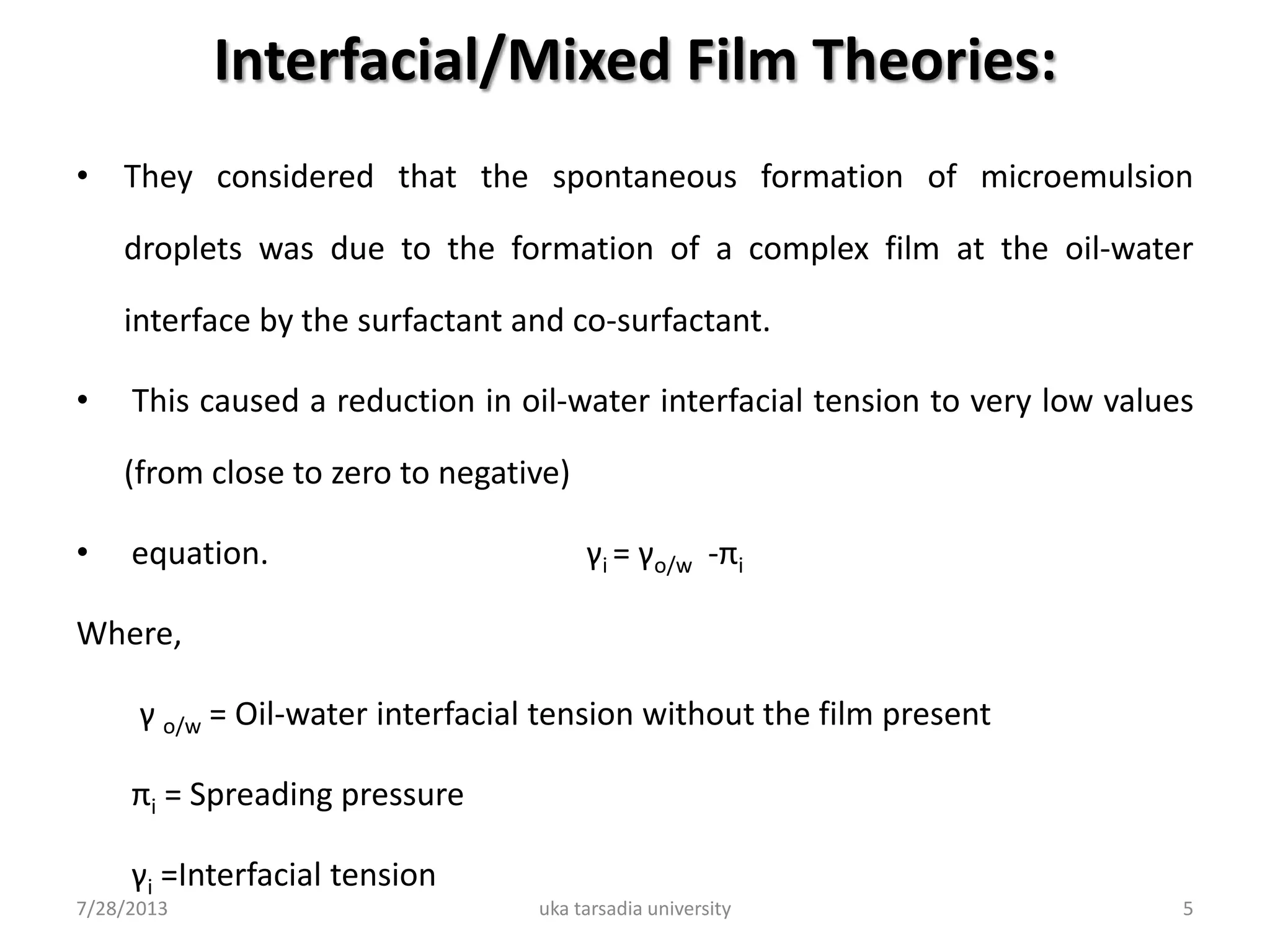
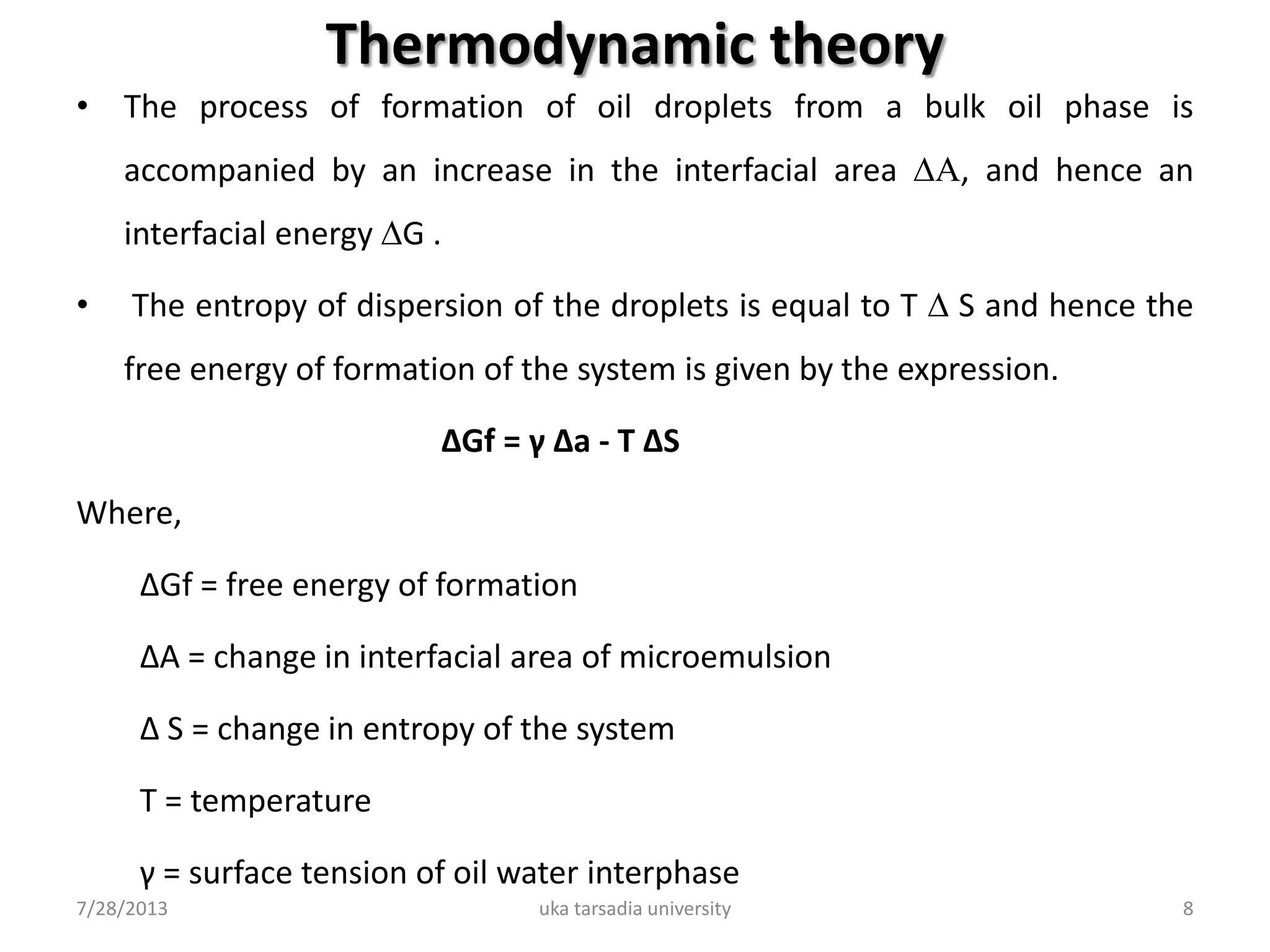
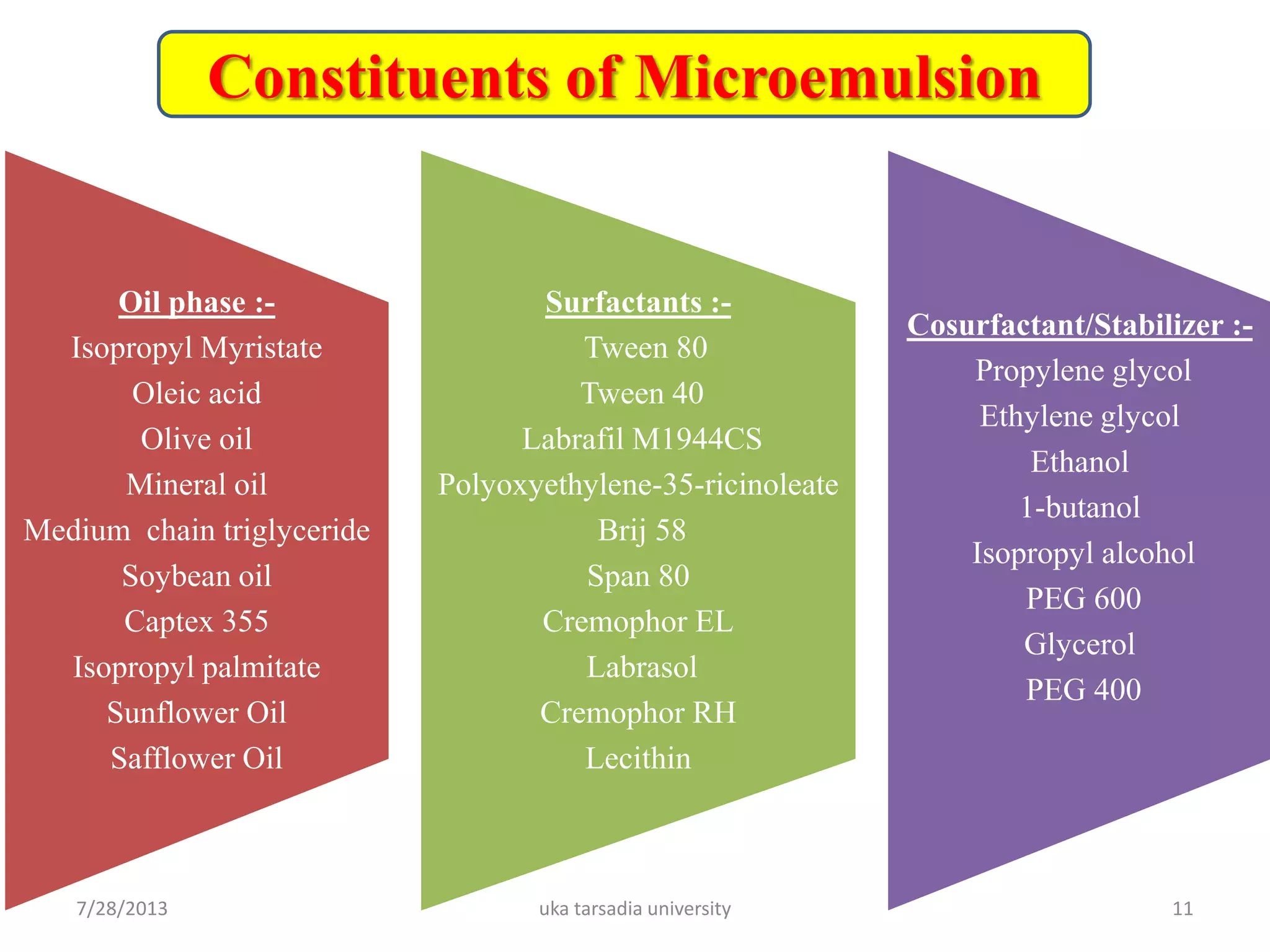
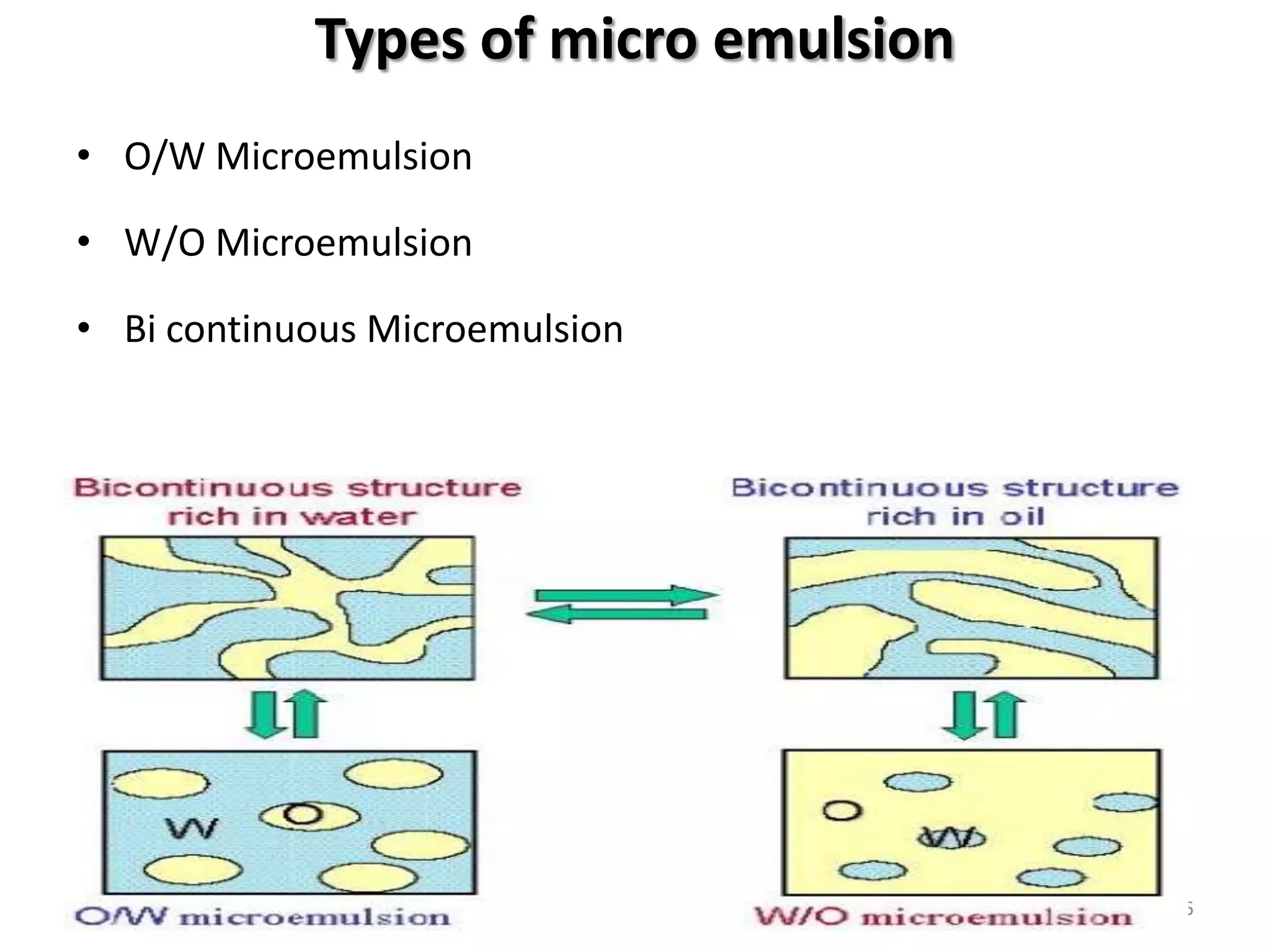
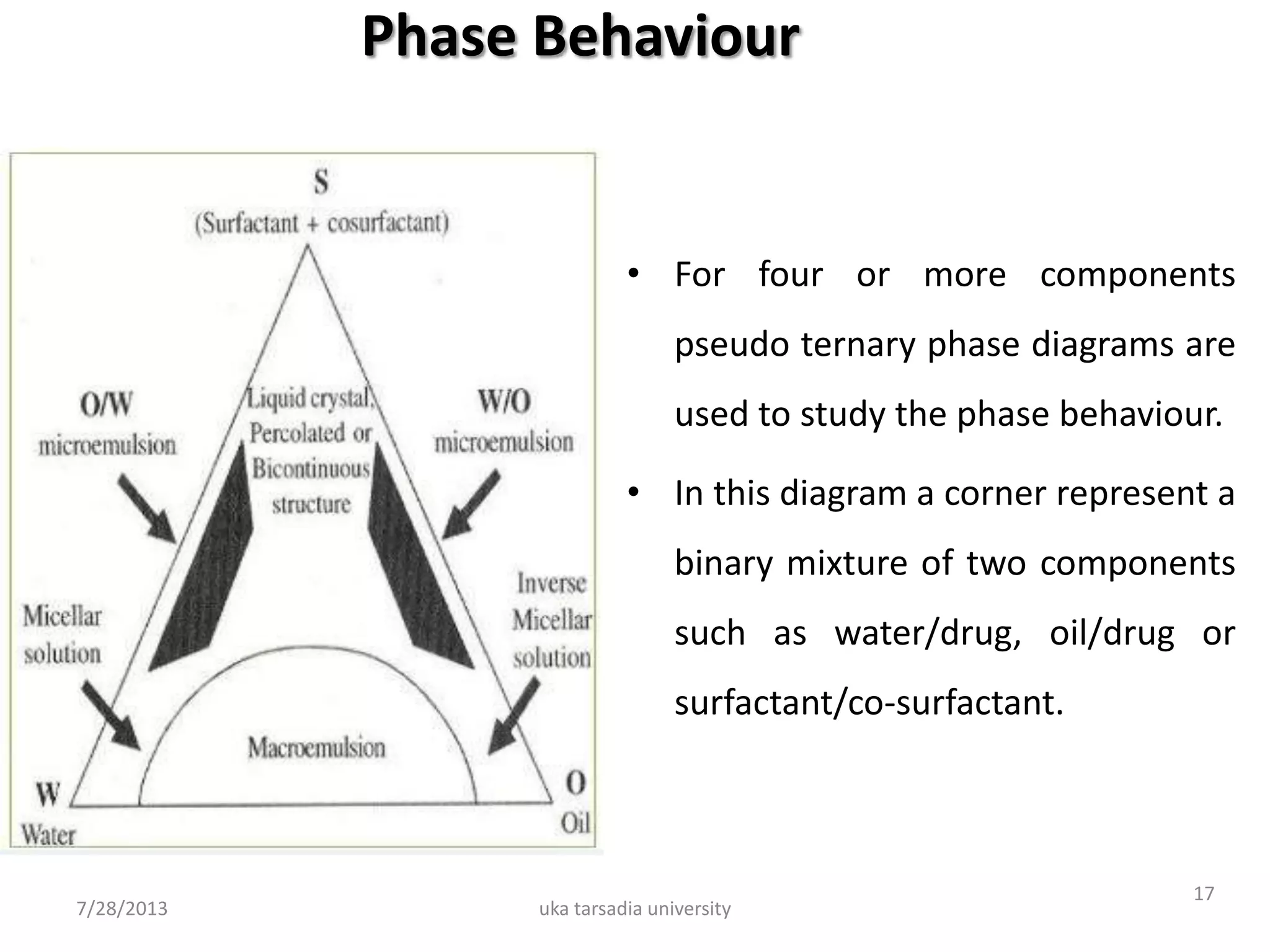
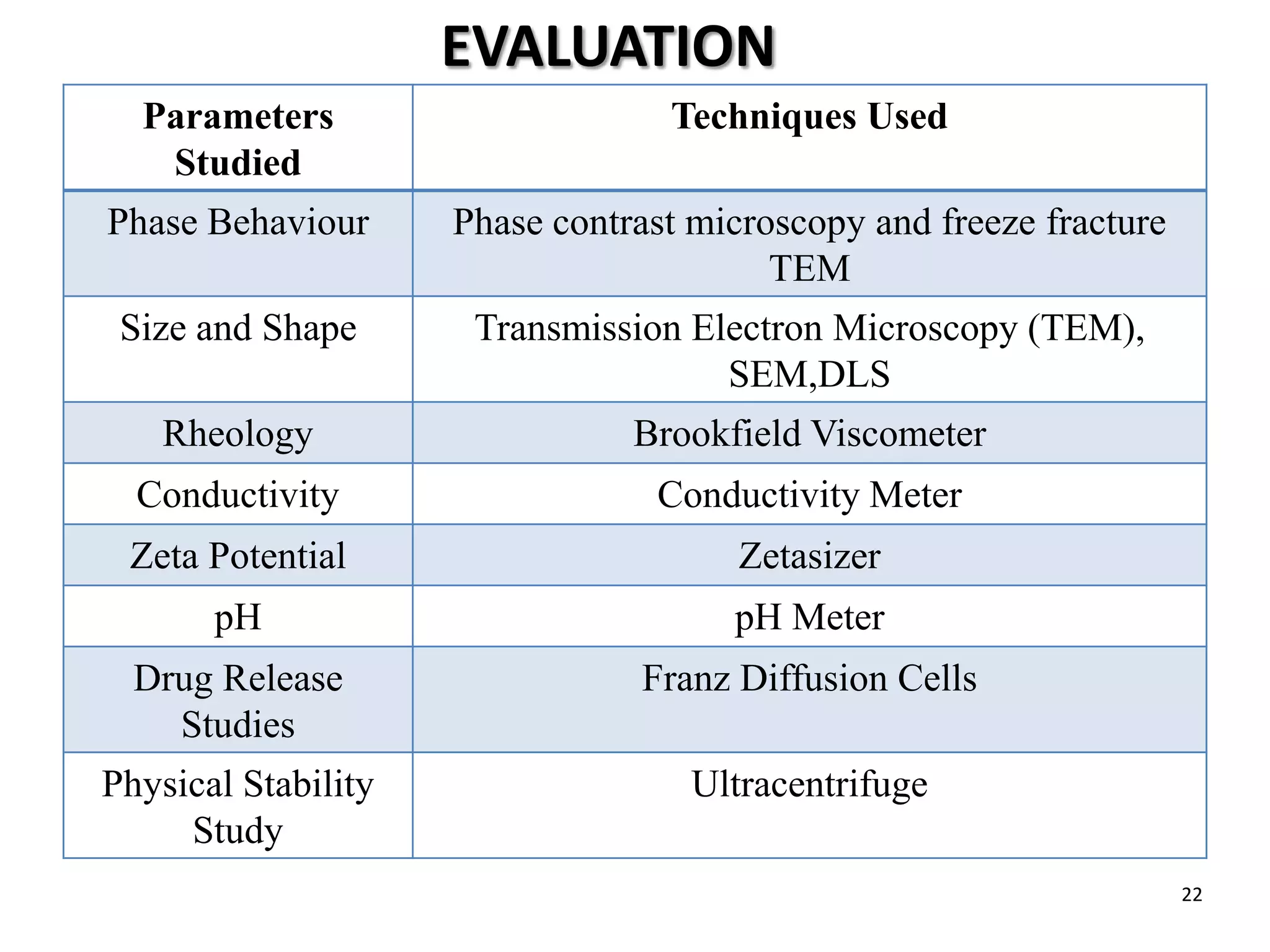
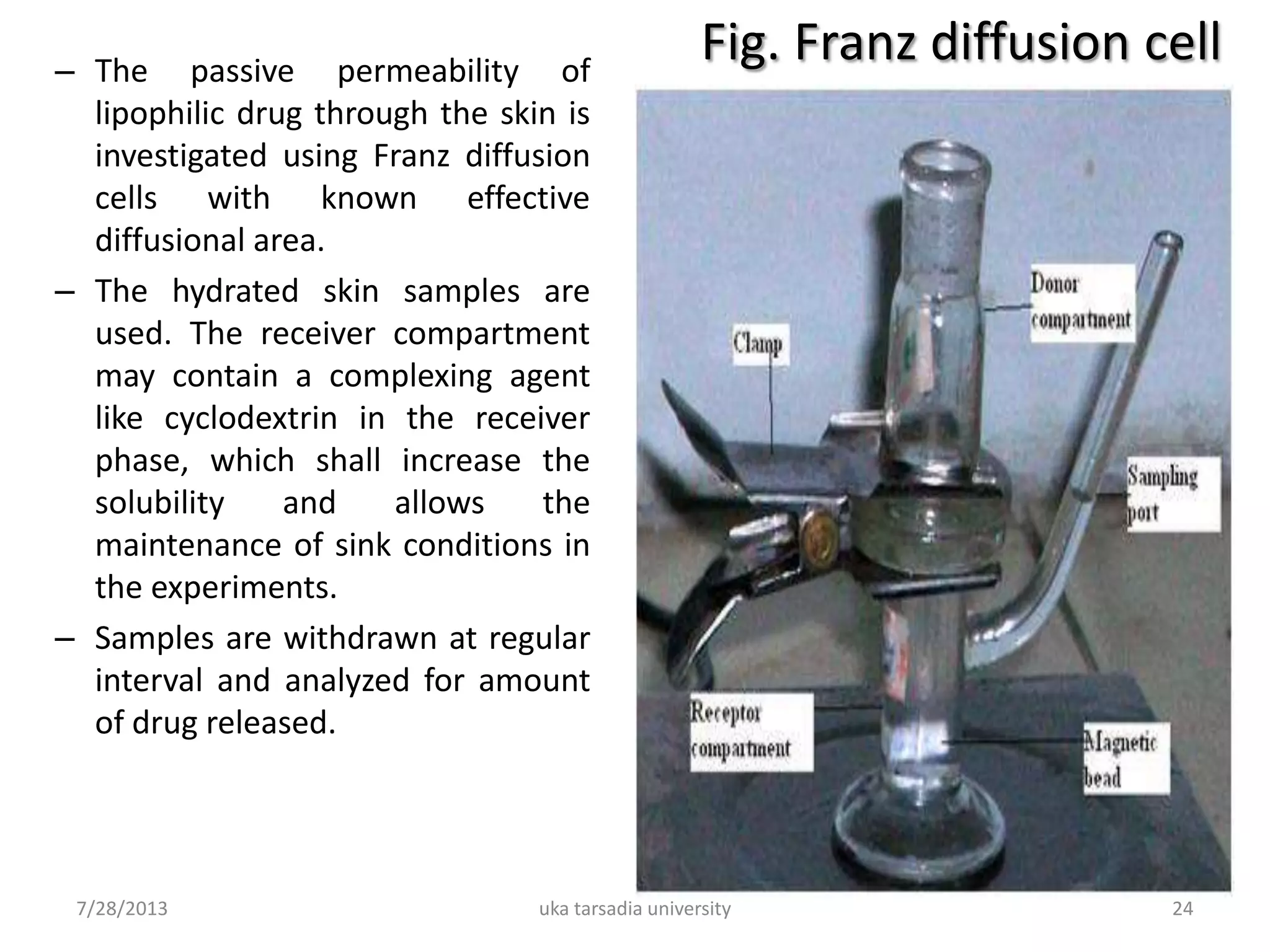
The document provides an overview of microemulsions including their basic principles, formulation, and evaluation. It defines microemulsions as thermodynamically stable dispersions of oil and water stabilized by surfactants and sometimes cosurfactants. The document discusses various theories of microemulsion formation and the role of the main components - oil, surfactant, and cosurfactant. It also describes methods for preparing and characterizing microemulsions and techniques for evaluating parameters like particle size, drug release, and physical stability.